Home page (in Russian) | Texts in English | Guestbook
|
• Alpha-Fetoprotein in Ontogenesis and its Assotiation with Malignant Tumors (1971) |
G. I. Abelev
Laboratory of Tumor Immunochemistry,
N. F. Gamaleya Institute for Epidemiology and Microbiology,
Moscow, USSR
|
I. Introduction: Major Steps in Development of the Problem II. Alpha-Fetoprotein Synthesis in Ontogenesis and Pathologic States. An Outline of the Phenomenon III. Alpha-Fetoprotein (AFP). Definition and Physicochemical Characteristics A. Definition and Identification B. Detection C. Isolation and Physicochemical Properties D. Further Studies IV. Site of AFP Synthesis in Ontogenesis A. Liver B. Yolk Sac V. The Dynamics of AFP in Ontogenesis A. The Early Embryonic Period B. From the Middle of Pregnancy to Its Termination C. The Early Postnatal Period VI. AFP Synthesis during Regeneration of the Liver A. AFP Synthesis during Regeneration of the Liver as a Model for Studying the Nature of the Phenomenon VII. AFP in Hepatocellular Cancer A. Site of AFP Synthesis in Cancer of the Liver B. Demonstration of AFP in the Blood in Cases of Liver Cancer and Other Diseases by the Method of Agar-Gel Precipitation C. Quantitative Aspects of AFP Production by Liver Tumors D. AFP Production by Hepatomas as Influenced by Etiologic and Pathogenic Factors VIII. Clinical Aspects of the Diagnosis of Liver Cancer IX. Alpha-Fetoprotein with Teratocarcinomas X. Concluding Remarks XI. Addendum References |
Ontogenetic studies on the protein composition of the blood serum have revealed a group of the so-called embryo-specific proteins, i.e., proteins peculiar to fetal or newborn sera and not present in blood of adult individuals. The first identified protein of this group was fetuin, an embryo-specific protein of calf serum discovered by Pedersen (1944). Next, Bergstrand and Czar (1956, 1957) described an embryo-specific α-globulin in human fetal serum.
At present, embryo-specific serum proteins have been demonstrated in each mammalian species studied in this respect (Gitlin and Boesman, 1966, 1967a; Masopust et al., 1971). Several types of such proteins have been shown to exist, belonging to α- and β-globulin serum fractions. Most characteristic among them is alpha-fetoprotein (AFP) which is represented, probably, in all mammalian species and exhibits similar physicochemical properties as well as common dynamics in ontogenesis. It is relevant to emphasize here that AFP is different from the classical fetuin of Pedersen (Kithier et al., 1968a).
Although antigenic relations between tumor and embryonic tissues were repeatedly investigated (see Day, 1965), the embryo-specific serum proteins have not been studied early by oncologists. Interest in the problem developed after the demonstration that transplantable hepatocellular carcinomas of the mouse were synthesizing and secreting into the blood an embryo-specific α-globulin subsequently identified as AFP (Abelev, 1963; Abelev et al., 1963a,b).
It was soon found that AFP appeared also with primary tumors of the liver and that its synthesis was characteristic not only of mouse but of rat hepatomas as well (Abelev, 1965a; Grabar et al., 1967).
In 1964 Tatarinov first detected AFP with human hepatocellular carcinoma and by 1966 he described six such cases (Tatarinov, 1964a; Tatarinov and Nogaller, 1966). The prospect appeared of an immunochemical diagnosis of liver cell cancer, based on AFP detection in the blood serum; this suggestion determined the main direction of subsequent studies.
Abelev et al. (1967a) and Uriel et al. (1967) confirmed and considerably extended observations of Tatarinov on the diagnosis of primary liver cancer in humans. Simultaneously, it was found by two independent groups that AFP appeared not only with liver cancer but also with malignant teratoblastomas of the testis and ovary, possessing elements of embryonic cancer (Assecritova et al., 1967; Abelev et al., 1967a; Masopust et al., 1967, 1968). AFP was not detected with tumors of other localization, including those metastatic to the liver, nor was it found with nonneoplastic liver diseases.
These early observations have been fully confirmed by subsequent studies, carried out in 1968-1970 on a large supply of clinical material which was obtained from countries with a high frequency of liver cancer – Senegal, Union of South Africa, Mozambique, Kenya, Uganda, Congo, and Indonesia – as well as from the USSR, England, France, USA, and Greece, where the disease rate is low. By these studies high specificity of the AFP test has been demonstrated as a means for differential diagnosis of hepatocellular cancer. The same conclusion was reached in the collaborative study undertaken by the International Agency for Research on Cancer in 1967-1969 (O'Conor et al., 1970). New practical problems became apparent; they were primarily those of early identification of AFP-producing tumors and of increasing the percentage of hepatocellular cancers and teratocarcinomas revealed by the AFP test.
In all the above-mentioned studies, AFP was detected by an agar-gel immunoprecipitation technique, which was, however, inferior to some other immunologic methods. It was natural to apply the most sensitive techniques of AFP determination for cancer diagnosis. The very first results with highly sensitive methods – aggregate hemagglutination (Olovnikov and Tsvetkov, 1969; Abelev et al., 1971), immunoradioautography (Abelev et al., 1971), and radioimmunoassay (Purves et al., 1970c) – showed that the specificity of the AFP test for liver cell cancer was far from being absolute. Low levels of AFP were found to occur during pregnancy and certain periods of noncancerous diseases of the liver. As a result, quantitative and dynamic aspects of the problem became important, especially in early cancer diagnosis.
Active studies on practical aspects of the problem have not been accompanied, however, with equally active basic investigations. And though the phenomenon itself – the synthesis of AFP in ontogenesis and by malignant tumors of the liver – has been described rather adequately, both in animals and man, its mechanism remains almost unstudied. Both the nature of control of AFP synthesis in ontogenesis and the reasons of resumption of its synthesis in tumors are to be determined.
In the present work, experimental and clinical evidence will be reviewed, the main attention being paid to approaches and prospects concerning research on the nature of the phenomenon and new aspects of its practical application.
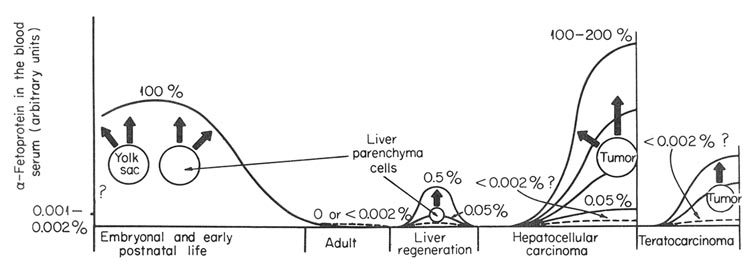
Before detailed analyses of different aspects of the subject are given, we would like to present the general scheme of the phenomenon. It is given in Fig. 1 with its proved and assumed elements indicated. In the embryonic period of ontogenesis, AFP is synthesized and secreted into the blood by cells of hepatic parenchyma and yolk sac. After birth, during the early postnatal period, AFP synthesis is carried out by liver cells only. In blood of healthy adult animals and humans AFP is not detectable by conventional methods. Some background serum level of this protein can, probably, exist in normal adult individuals, but it should not exceed 0.001% or 0.002% of its maximal level in fetal serum. AFP synthesis is resumed in animals during regeneration of the liver and seems to be carried out by cells of hepatic parenchyma. In this case, the synthesis has reversible character. Its intensity varies greatly in different species. It is not known whether liver regeneration can proceed without the AFP synthesis.

Fig. 1. Schematic representation of AFP synthesis in normal development and pathologic states. Solid line, serum AFP level, in arbitrary units. Broken line, expected AFP level. ?, not known.
Synthesis of AFP is resumed during development of hepatocellular carcinomas or germinal tumors with elements of embryonic cancer, the production being permanent. In both neoplastic diseases, tumor itself is the site of synthesis, although additional proof may be required in case of teratocarcinomas, where cell elements responsible for AFP synthesis have not yet been identified. The level of the synthesis in "AFP-positive" tumors varies within very broad limits, more than 1000-fold. Some hepatomas and teratocarcinomas do not produce AFP, or rather produce it in undetectable amounts.
These are the main features of the phenomenon. As to its nature, which remains not yet elucidated, three possibilities have been discussed in the literature:
1. AFP synthesis may be an inducible process, similar to induction of certain enzymes in the liver by appropriate substrates or hormones. In tumor, a fortuitous and nonregular inactivation takes the place of a corresponding repressor (Grabar et al., 1967).
2. AFP formation is believed to be a property of specialized cells of liver parenchyma. These cells are the final stage of the peculiar branch of liver stem cell differentiation. The alternative pathway results in the formation of hepatocytes producing the "adult" serum proteins. The dynamics of AFP in ontogenesis and appearance in tumors, according to this view, is a reflection of the presence of the hypothetical AFP-forming cells and changes in their number (Abelev, 1968).
3. Only a certain stage in the hepatocyte development is considered to be endowed with the potential for AFP synthesis. Hepatocytes of earlier and later stages of differentiation do not synthesize AFP. In hepatomas a dedifferentiation of the hepatocyte ("retrodifferentiation") takes place, which results in an "AFP-positive" tumor if it corresponds to the "AFP-producing" stage (Uriel, 1969).
In subsequent discussions we shall consider individual aspects of the above points, with special attention to unsolved problems.
To reveal and identify specific proteins in the embryonic serum, immunochemical methods are commonly used. An antiserum against fetal serum absorbed with a homologous adult serum serves as a specific reagent for embryo-specific antigens in Immunoelectrophoresis or immunodiffusion. Depending on the animal species, such antisera reveal from one to three components in fetal sera. AFP is the most characteristic representative of this group and has been reported for 18 mammalian species examined in this way (Gitlin and Boesman, 1966, 1967a; Maso-pust et al., 1971).
According to the definition proposed by WHO-IARC experts, AFP is the first α-globulin to appear in mammalian sera during development, and the dominant serum protein in early embryonic life. It reappears in the adult serum during certain pathologic states, primarily with hepatocellular carcinoma (Annual Report of International Agency for Research on Cancer, 1969).
AFP's from different animal species exhibit clear-cut cross-reactions. Taking advantage of this property, one can distinguish AFP from other embryo-specific proteins by demonstrating cross-reactivity with a reference AFP preparation, for instance that of rat or man.
Table I summarizes present knowledge of the physical, chemical, and immunological properties of AFP's, distinguishing this protein class from other embryo-specific proteins, and the unified terminology proposed for them. The corresponding table composed by the WHO-IARC expert group has been taken as a basis (Annual Report of International Agency for Research on Cancer, 1969). The table includes AFP of man and of most studied animals.
TABLE I
Alpha-Fetoprotein and Other Embryospecific Serum Proteins.
Terminology, Physico-Chemical and Immunochemical Properties a
Additional information concerning other animals may be found in the reports of Tatarinov and Afanassieva (1965), Gitlin and Boesman (1966, 1967a), and Masopust et al. (1971).
Methods of AFP determination should be briefly discussed here, since the evaluation of experimental and clinical conclusions is essentially dependent on their specificity and sensitivity.
AFP in fetal and cancer patient sera can be assayed by a variety of analytical electrophoresis procedures: on paper, cellulose-acetate strips, and most clearly in starch and polyacrylamide [Table I; Fig. 2(a)]. Sensitivity of these methods is not high since they depend on zone densities detectable by staining. Their specificity is fair in the case of sera possessing characteristic patterns of component distribution, but it seems almost impossible to identify AFP in tissue extracts with these methods.
Much more sensitive and specific are the immunochemical methods – Immunoelectrophoresis and agar-gel precipitation – especially when a specific anti-AFP serum is employed. The agar-gel precipitation test with single or double diffusion is a standard procedure of AFP determination providing for quantitative assay and possessing a sensitivity level of 1-3 /xg./ml. AFP with absolute specificity (Khramkova and Abelev, 1961; Purves et al, 1968).
This level of sensitivity appears, however, not to be sufficient for some experimental and clinical investigations. New and highly sensitive tests for AFP determination have been suggested recently: aggregate-hemagglutination (Olovnikov, 1967; Olovnikov and Tsvetkov, 1969), indirect immunoradioautography (Rowe, 1969, 1970a; Abelev, 1971; Elgort and Abelev, 1971) and radioimmunoassay (Purves et al, 1970). In modifications used, the sensitivity limit of these tests is about 0.05 μg./ml. AFP, that is 25-50 times lower than with agar-gel precipitation.
The essence of these methods is as follows:
1. The aggregate-hemagglutination test may be considered as "reverse hemagglutination." The erythrocytes are conjugated with a pre-polycondensed anti-AFP-serum which renders them agglutinable with a corresponding antigen. Agglutination may be observed with as little antigen as thousandth portions of micrograms per milliliter, but since first dilutions of the antigen assayed are not taken into consideration (up to 1:10 or 1:15), the practical sensitivity limit is about 0.05 μg./ml. The reaction does not possess resolving power and its specificity depends on that of the antiserum used.
2. The radioimmunoassay is based on competition for corresponding antibody between the AFP in the assayed sample and reference 125I-labeled AFP preparation. The antigen-antibody complex is precipitated with antibodies against the immunoglobulin used in the reaction. From a displacement of 125I-AFP in the test sample relative to the control, one can judge the presence of AFP in the examined sample. Sensitivity of the method is extremely high (for some antigens to nanograms), while its specificity depends on the degree of purity of the reference AFP-anti-AFP system.
3. The indirect immunoradioautography test is a modified gel-precipitation reaction. Sensitivity of gel precipitation is known to increase directly with dilution of the antiserum and reference antigen comprising the test system. The precipitation zone formed by highly diluted reagents becomes invisible, however. In the modified test, visualization of the invisible precipitation band is accomplished with anti-γ-globulin antibodies labeled with 131I or 125I followed by autoradiography of agar plates. The method is about 15 to 30 times more sensitive than agar gel precipitation, and preliminary concentration of examined samples allows an additional 2- or 3-fold increase in sensitivity. As distinct from the above two methods, the immunoradioautography test is absolutely specific.
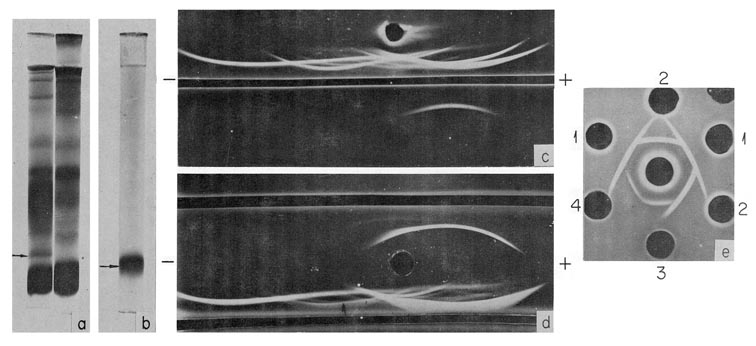
Fig. 2. Characteristics of human AFP. (a) PAAG electrophoresis pattern of fetal (left) and adult (right) human serum. AFP position is indicated by the arrow, (b) PAAG electrophoresis pattern of purified AFP. (c) Immunoelectrophoretic pattern of a fetal human serum (above) and of the purified AFP (below). Developed by antiserum to fetal human serum, (d) Immunoelectrophoretic pattern of a fetal human serum, developed by the antiserum to fetal human serum (bottom trench) and by unabsorbed anti-AFP serum (upper trench), (e) Cross-reactions of human AFP (wells 1) with that of calf (wells 2) mouse (well 3) and rat (well 4). In the central well rabbit antihuman AFP serum. (According to Gusev and Jazova, 1970a,b and Jazova and Gusev, 1971.)
Table I includes the available information on the purification methods and physicochemical characteristics of AFP's. The molecular weights of AFP's are approximately 65,000-70,000; they contain only traces of carbohydrate. The electrophoretic mobility differs depending on animal species: it is similar to that of albumin in man, monkey, or rat. Mouse AFP is located in the α2-globulin fraction while dog AFP is in the effraction (Tatarinov and Afanassieva, 1965; Masopust et al., 1971).
Table I reflects certain progress in the study of different animal AFP, which has been obtained due to development of several effective methods for isolation and purification of this protein.
In physicochemical properties AFP is very close to serum albumin. AFP is almost inseparable from the albumin by agar-gel or paper electrophoresis of fetal sera of man, monkey, or rat. Even using polyacrylamide-gel electrophoresis, one cannot obtain complete preparative separation between the two proteins in human and rat sera. All the effective methods proposed for AFP purification employ a combination of physicochemical and immunochemical approaches. Thus, in the initial method of AFP purification, immunofiltration, the separation from electrophoretically inseparable impurities was obtained by a counterelectrophoresis of the antigen mixture through a "filter" composed of antibodies against the impurities (Abelev and Tsvetkov, 1960; Abelev, 1965a,b). Stanislawski-Birencwajg (1967) purified AFP from fetal sera using adsorption of all "adult" antigens on an immunosorbent prepared from polycondensed antiserum against adult rat serum. Nishi (1970) and Nishi et al. (1971) obtained pure AFP from its precipitate with a corresponding antibody.
The most simple and effective method of purification seems to be that proposed by Gusev and Jazova (1970a), which combines preparative separation in polyacrylamide gel (PAAG) with a final purification step employing antibody. According to this procedure, AFP is first isolated from fetal serum by two cycles of preparative PAAG electrophoresis. The AFP fraction contains some serum albumin, and by addition of the equivalent amount of antialbumin antibody, followed by PAAG electrophoresis, a highly purified AFP preparation is obtained [Fig. 2(b,c)].
The method has been successfully used for isolation of human, rat, and mouse AFP. Pure AFP preparations have enabled us to find out the reliable physical and chemical characteristics and particularly to demonstrate the similarity of AFP from experimental animals and man.
Of no less importance is the use of pure AFP for the preparation of potent, strictly specific antisera. Thus, using immunization of rabbits into lymph nodes with purified AFP, very potent AFP antisera have been obtained (Gusev and Jazova, 1970b). Serum antibody titers reached 1:500 in immunodiffusion, with the immunizing antigen dose as little as 0.5 mg [Fig. 2(d)]. Potent AFP antisera clearly reveal cross-reactions between AFP's from distant species, such as man, cow, mouse, and rat [Fig. 2(e)]. The occurrence of cross-reactivity is good evidence in favor of the assumption that AFP's of the experimental animals and man are indeed homologous proteins, as are their serum albumins and immunoglobulins.
Basic information on physical, chemical, and structural peculiarities of AFP presents more interest as an approach to understanding control of its synthesis. The available evidence does not provide a basis so far for any suggestions regarding the control mechanism of AFP production. It would be very helpful to have information on the submolecular structure of AFP.
First of all, one can expect that AFP is replaced during development by some "adult" protein with a similar function in the organism. Since in physicochemical properties AFP is close to serum albumin and seems to replace it (at least on electrophoreograms) at the earliest stages of development when the albumin is present only in trace amounts, it can be suggested that serum albumin is the counterpart of AFP, just as "adult" hemoglobin displaces the embryonic type of hemoglobin.
It would not be unreasonable to suppose that these proteins are close in function and homologous in origin. On this basis, similarity in their primary structure can be expected. It would be quite desirable, therefore, to know the primary structure of the polypeptides constituting both proteins or, at least, to compare their peptide maps (fingerprints).
Second, by comparing replacement of embryonic hemoglobin with the adult type protein, one might think of partial alterations in the sub-molecular structure of AFP rather than of total discontinuation of its synthesis in the adult organism. In this respect it is necessary to know first of all, whether AFP consists of one or more peptide chains. No studies of this kind have been reported so far.
Both of the above questions can be answered in the near future, since availability of simple and effective isolation and purification methods provides full opportunity for such studies.
Detection of genetic variants of AFP would be of indisputable interest for elucidating mechanisms controlling the synthesis of this protein. It would provide approaches for determining the number of genes controlling the synthesis, their localization and expression at cellular and organism levels. Very important in this respect are the reports of Purves et al., (1969) and Portugal et al. (1970) who detected by electrophoresis a rare "slow" variant of human AFP, significantly different in mobility from the usual type. Genetic analysis would undoubtedly be interesting in this case, as would establishment of the biochemical basis of this property – whether it results from a deviation in primary structure of the protein or from some secondary aggregation defect, or from complex formation with other compounds.
From the point of view of genetic analysis, detailed physicochemical and immunochemical investigation of AFP's from various mouse and rat lines is also promising. Interline and interspecies immunization might reveal line-specific determinants similar to allotypes in immunoglobulins. That would provide the basis for genetic analysis.
It is known that lost or greatly reduced ability to form certain proteins can also be a genetic trait. Analyzing composition of sera from individual human fetuses, Tatarinov (1965b, 1968) found that about 2% of the fetal sera contained no AFP. The age of the fetuses was 24 and 27 weeks and it is possible to think of changed dynamics of AFP formation, namely, of its premature disappearance from serum. Be that as it may, these observations require confirmation and a more detailed analysis.
Finally, one can mention the problem of similarity or difference between the embryonic AFP and that of tumor origin and of AFP's produced by various tumors. Detailed analysis of the protein from individual patients and individual fetuses might reveal possible "abnormalities" or at least heterogeneity in AFP. The latter is an important indication in the analysis of control of protein synthesis, as it has been shown, for example, in immunoglobulin studies.
Human and mouse AFP's behave as single proteins both in electro-phoretic and sedimentation analysis. AFP of the rat, however, reveals a definite double zone in starch gel (Wise et al., 1963; Kirsh et al., 1967; Gusev and Jazova, 1970a; Nishi et al., 1971). It is not known what this "bifurcation" is related to and whether or not it is developed by AFP synthesized by rat hepatomas.
Considerable material has accumulated on electrophoretic behavior of AFP's from individual patients (Purves et al., 1969; Portugal et al, 1970). No significant differences have been observed in their electrophoretic patterns. The above-mentioned "slow" variant was detected in less than 2% of cases. This seems to indicate that human AFP possesses no important heterogeneity.
Comparison of AFP's from sera of individual fetuses and patients showed very small differences between the groups in electrophoretic mobility in agar gel and PAAG. Fetal AFP's possessed a mobility which was only ≈5% higher than that of patient AFP's (Gusev et al., 1971a). In this particular study, fetal sera of Europeans and patient sera of Africans were used. The possibility that the above differences were race-determined is not excluded.
Summarizing this section, we may conclude that AFP of different mammals is similar in physicochemical properties and contains common antigenic determinants. Its physicochemical study is fairly satisfactory, but characteristics which could be used in investigation of mechanisms controlling its synthesis are still lacking. Search for such characters is the significant task in studying properties of this protein.
It may be considered established at present that during the embryonic period of ontogenesis, AFP is synthesized by the liver parenchyma cells and yolk sac cells. Two kinds of evidence support this statement: in vitro synthesis of AFP by the embryonic liver and yolk sac, and specific localization of AFP in cells of liver parenchyma and yolk sac.
The synthesis of AFP by embryonic liver in vitro has been demonstrated by immunoradioautography technique according to Hochwald et al. (1961). Surviving pieces of embryonic or newborn liver of mice, rats, and man actively incorporated 14C-amino acids into AFP. The labeled protein was determined by autoradiography in the precipitation band obtained in immunoelectrophoresis or agar double diffusion with a monospecific anti-AFP serum. The incorporation was specific, since no 14C-amino acids were found included in precipitates of proteins, not synthesized but present in the system, and no incorporation of the label resulted in AFP following incubation of other embryonic organs: spleen, kidney, lung, placenta,¹ intestine, stomach, heart, and brain (Abelev, 1965a; Wise and Oliver, 1966; Abelev and Bakirov, 1967; Gitlin and Boesman, 1967b; Van Furth and Adinolfi, 1969; Uriel, 1969).
|
¹ In experiments of Van Furth and Adinolfi (1969), AFP synthesis was reported for human placenta in 2 cases of 7. This was not noted in other studies (Wise and Oliver, 1966; Gitlin and Boesman, 1967b) and no explanation can be proposed at present for the discrepancy. |
Further support of the above statement has been obtained from experiments with organ cultures of mouse embryonic liver. Such cultures actively synthesized AFP, albumin, and transferrin and secreted them into the medium in the course of 2 or 3 weeks of cultivation (Luria et al., 1969).
Accumulation of AFP went on with multiple renewals of the medium, so that the total amount of AFP greatly exceeded that introduced with the explant. Besides, the occurrence of AFP synthesis was confirmed by incorporation of 14C-amino acids into the protein.
Thus, AFP formation by the liver of the embryo has been sufficiently well documented. But embryonic liver is not homogeneous in cell composition; moreover, it is a place of active hematopoiesis, and it remained unknown which liver cells were responsible for AFP synthesis and whether or not the hematopoietic tissue could be the site of its formation. Of course, the specific relation of AFP to hepatocellular cancer, when tumors themselves produced AFP, indicated, although indirectly, that the AFP formation in normal conditions was a function of liver parenchyma cells.
More directly, this was supported by immunofluorescence studies of AFP in sections of different embryonic organs and of cultured embryonic liver. Application of this method for AFP localization had encountered serious difficulties for a long time, since the usual cryostat technique, combined with various fixing reagents, either failed to reveal AFP in sections at all or produced obscure and poorly reproducible results. Only in one study, by Gitlin et al. (1967), was AFP shown rather definitely to be located in the embryonic liver parenchyma and yolk sac.
Much more reliable results were obtained when the paraffin technique of Sainte-Marie was used (Sainte-Marie, 1962; Hamashima et al., 1964). Engelhardt et al. (1969) using this method showed specific localization of AFP in the liver parenchyma cells of mouse and human embryos (Fig. 3) with concurrent absence of this protein from the liver hematopoietic tissue cells, bile ducts, and Kupfer's cells.²
|
² Erythroblastic localization of AFP was observed by Dufour et al. (1969) in the cell suspension of embryonic rat liver. They reported no data about the AFP localization in hepatocytes. |
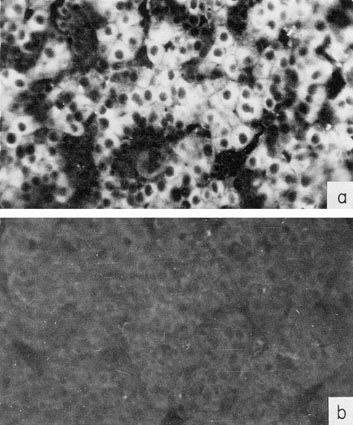
Fig. 3. Localization of AFP in human embryo liver by immunofluorescence, (a) A section of 6-weeks-old embryo liver, treated by antibodies to AFP, and then by fluorescein-conjugated antibody to rabbit γ-globulin. (b) The same as in (a) but anti-AFP serum was neutralized by an equivalent amount of purified AFP. X40. (According to Engelhardt et al., 1969.)
In organ cultures of the liver, also, the AFP-containing cells were identified, both in sections and in total preparations (Basteris et al., 1971). These were hepatocytes, which, as a rule, were distributed in the tissues as trabecules (Fig. 4).
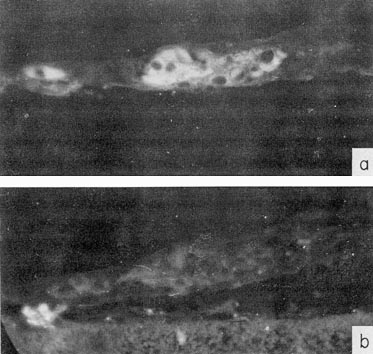
Fig. 4. Localization of AFP in organ cultures of the mouse embryo liver by immunofluorescence, (a) Culture section treated by rabbit antibodies to AFP and then by fluorescein-conjugated antibody to rabbit γ-globulin. (b) Serial section treated as in (a) but anti-AFP was neutralized by purified AFP. X40. The membrane filter supporting the cultures is seen in (b). (According to Basteris et al., 1971.)
Thus, the conclusion that the liver parenchyma cells are a site of AFP synthesis in normal ontogenesis seems to be well substantiated by the above evidence.
The yolk sac has been much less studied in this respect. Gitlin and Boesman (1967b) demonstrated that this organ of rats incorporates 14C-amino acids into AFP during short-term cultivations in vitro. Besides, these authors have detected AFP in cryostat sections of the yolk sac (Gitlin et al., 1967).
More detailed investigation of the yolk sac would be desirable.
Detailed investigation of AFP dynamics in ontogenesis is of principal interest. It may throw light on still-unknown functions of the protein and on mechanisms controlling its synthesis.
Thus far the problem has been studied rather irregularly. Most information pertains to the middle and terminal periods of pregnancy or to the time when AFP synthesis is being stopped, while the period when the synthesis is established, early in ontogenesis, has been studied very little.
Conclusions about the dynamics of AFP and other serum proteins are exclusively based on data from electrophoretic and immunodiffusion analysis. No attempts have been made to measure the rate of AFP synthesis in comparison with other serum proteins. Neither were any correlations found between AFP production and other features of the developing organism. Studies on the cellular basis of AMP formation have just been started. All that still purely phenomenological information is absolutely necessary to proceed to the analytical stage of research concerning the function of AFP and regulation of its synthesis.
There are no data so far about the exact time in ontogenesis when AFP first appears, nor about the initial site of its production.
Some idea of serum composition in the early embryonic period may be obtained from data relating to its termination, that is to the sixth or jighth week of development in man and to ≈2 weeks in rats. The protein composition of sera at that time is very distinct from the adult serum, or even from sera of more advanced fetuses.
AFP is the dominating protein in sera of mice and rats, while albumin and transferrin – the main components of adult sera – are present in trace amounts (Fig. 5a), (Pantelouris and Hale, 1962; Wise et al., 1963; Abelev, 1965a,b; Kirsh et al., 1967; Perova, 1969). In human 8-week-old embryos, although albumin prevails over AFP, both proteins are present n comparable concentrations (Fig. 5b) (Galdo et al., 1959; Andreoli and Robbins, 1962).
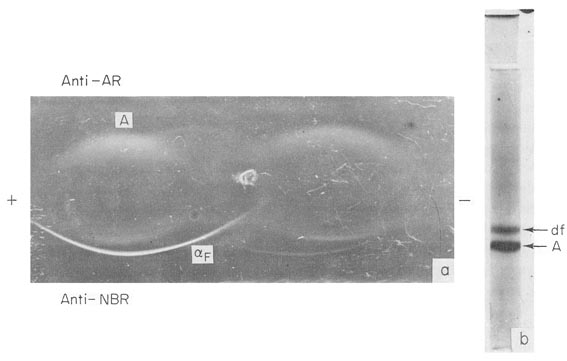
Fig. 5. AFP in early embryonal sera, (a) Immunoelectrophoresis of a rat embryo serum. (The weight of the embryo was about 0.15 gm.) Anti-AR, rabbit mtiserum to adult rat serum; Anti-NBR, rabbit antiserum to newborn rat serum; αF-AFP, A-albumin. (b) PAAG electrophoresis of human 8-week-old embryo serum. αF – AFP, A – albumin.
Engelhardt3 (1970) detected AFP in fluids of a 4-week-old human embryo, its concentration being much higher than that of the serum albumin.4 It seems therefore, that AFP in humans, like that in mice and rats, is also the dominating protein during the earliest stages of development.
|
3 Gamaleya Institute, Moscow. |
It is possible that true proportions between AFP, albumin, and transferrin in the "early" sera are masked by penetration into the fetal blood of mother serum proteins, already when in small amounts. In this respect, measurements of serum protein synthesis, accomplished by the embryo during this period, according to incorporation of 14C-amino acids, would be extremely important, as would determinations of labeled proteins penetrating from the blood flow of the mother. The use of autoradiography of PAAG electrophoreograms (Herrich and Lawrence, 1965) would permit exact answers to these points.
By comparing the "early" sera with those from later stages, it is clearly indicated that an appearance of new components, peculiar to adult serum, is continuously taking place in the embryo, as well as an increase in albumin and transferrin concentrations (see Wise et al., 1963). It is obvious that the most "early" sera studied do not correspond to the first stage of the process, and it would not be unexpected if the initial "primary" sera contained AFP not only as principal but as the only protein component synthesized by the embryo.
It is possible that initially AFP is selectively synthesized in the yolk sac, which provides for its high concentration in embryonic sera with only trace amounts of the albumin. As soon as hepatocytes begin functioning, a rapid accumulation of the albumin would begin, along with a further increase in AFP. To test this possibility, AFP- and albumin-forming cells in the yolk sac and liver should be studied by immunofluorescence techniques during the earliest stages of embryonic development.
Should the "early" embryonic AFP be formed only by the yolk sac, it would be interesting to compare its properties with that of liver-produced AFP which can be obtained in early postnatal life or in animals with regenerating liver.
This period has been more completely investigated in man and animals (Bergstrand and Czar, 1957; Galdo et al., 1959; Muralt and Roulet, 1961; Pantelouris and Hale, 1962; Andreoli and Robbins, 1962; Wise et al., 1963; Tatarinov, 1965; Tatarinov et al., 1967; Masopust, 1966; Masopust et al, 1967; Van Furth and Adinolfi, 1969; Perova, 1969; de Nechaud and Uriel, 1971; Zizkovsky et al., 1971). Figure 6 presents the AFP and albumin levels in sera of fetuses and newborns for man (a,b) and rat (c), the most studied species in this respect. In spite of the great difference in duration of the process and the different position of birth on the curves, the latter are of similar type. An initial rise of AFP level up to a maximum of about 4 mg./ml., some period of plateau, changing later into a period of AFP decline, until it completely disappears – are observed in both cases. It is not known what parameters of the developing embryo determine the character of the curve: its maximum turning point, and cessation of the AFP synthesis. It is certain, only, that neither the duration of development, so different in absolute values for man and rat, nor the time of birth constitute the determining factors for the curve of AFP dynamics.
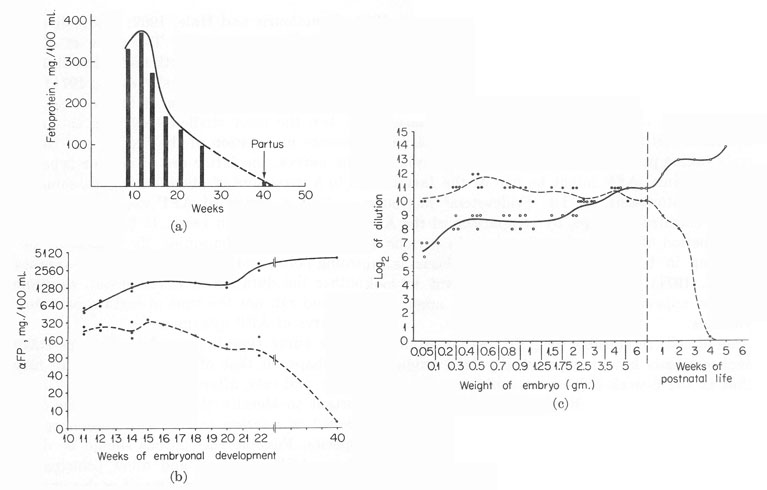
Fig. 6. The dynamics of AFP and serum albumin in the ontogenesis of man and rat. (a) AFP in human fetal sera. (From Masopust et al., 1967.) (b) AFP and serum albumin in human fetal sera. (According to a report of Van Furth and Adinolfi, 1969.) Solid line, serum albumin. Broken line, AFP. (c) AFP and serum albumin in rat fetal sera. (According to Perova, 1969.) Solid line, serum albumin. Broken line, AFP.
Evidently, the curve type is dependent on some stage of maturation of the fetus, perhaps on that of its liver, which in man occurs before birth and in mice and rats, afterward (Zizkovsky et al., 1971). It would be most important to identify this character of the developing embryo, equally positioned in relation to the AFP dynamics curve in different mammalian species. Possible correlations should be determined, for this purpose, between AFP synthesis and other principal functions of the liver or of other organs of the fetus. One of such correlations may be the obvious coincidence with the embryonic hematopoiesis. Both processes are successively taking place in the yolk sac and liver, and both terminate at about the same time. AFP might be one of the factors essential for the embryonic hematopoiesis, as Dr. Fridenstein5 has suggested. The question may be clarified, perhaps, by comparison of the AFP dynamics curves with the period of hepatic hematopoiesis in various animals, significantly differing in time when the AFP synthesis is stopped (see Zizkovsky et al., 1971). Of course, it is important to look for other characters, whose appearance and disappearance might correlate with the AFP dynamics.
|
5 Gamaleya Institute, Moscow. |
Distribution of cells responsible for AFP production during the period of its maximal synthesis have not practically been studied. There is an indication that in the 6-week-old human embryo and in newborn mice, the overwhelming majority of liver parenchyma cells contain AFP (Engelhardt et al., 1969). To investigate the cellular basis for the production of AFP, albumin, and transferrin in the developing embryo seems to be an exciting as well as quite feasible task. The paraffin technique of Sainte-Marie, which is excellently suitable for cellular localization of serum proteins and permits use of serial sections, provides practical opportunities for detection of several proteins in the same tissue regions (Engelhardt et al, 1971).
Such an investigation would answer the question whether the production of AFP and that of "adult" serum proteins takes place in the same hepatocytes, or if the liver may be considered as a mosaic of cells, specialized in the synthesis of one or another protein. It is absolutely necessary to know this for investigating the mechanisms which regulate serum protein synthesis during development.
The termination of AFP synthesis is a stage of special interest since it is during this period that the controlling factors come into force. Nothing is known so far about their nature. It has been shown only that the intensity of proliferation of hepatic tissue does not correlate with AFP synthesis (Abelev et al., 1967b). A temporary arrest of mitosis in the liver of 1- to 7-day-old rats (McKellar, 1949) was not accompanied by cessation of AFP synthesis, and on the other hand, termination of the synthesis (fourth week) took place during intensive proliferation of hepatocytes. Thus, the onset of division in hepatocytes or its decline does not seem to be a factor regulating AFP synthesis.
No answer can be given at present to even one of the principal questions, whether a gradual decline of ATP synthesis takes place in all hepatocytes or if only the number of AFP-forming cells decreases. In other words, is it the intensity which is controlled in the AFP synthesis taking place in all hepatocytes or, rather, is the number of cells involved the object of control?
We made an attempt to answer this question by determining AFP, using immunofluorescence, in sections of the mouse liver during the early postnatal period (Abelev, 1971; Shipova et al., 1971). A notable heterogeneity in fluorescence was observed in newborn or 1- or 2-day-old mice.
The heterogeneity markedly increases, beginning from the third to the fifth day of postnatal life. It consisted in regular grouping around the central veins of brightly luminescent cells, clearly differing from the majority of cells in the lobe, which possessed a homogeneous unelevated level of fluorescence, exceeding, however, that of controls (Fig. 7). This may indicate either a difference in the level of ATP synthesis between different liver cells, or that the synthesis is accomplished by a limited number of cells regularly situated in the lobe. With fading of the synthesis, the number of brightly luminescent islets in the lobe diminished, together with the number of cells composing them. By the time AFP synthesis ceased – the fourth week of postnatal life – no luminescent cells in the liver were observed. We are inclined to think, therefore, that the decrease in AFP level is due to a smaller number of cells synthesizing AFP rather than to fading of a process taking place in all cells of the liver. The suggestion requires a further study with both in vivo systems and organ cultures of the liver.
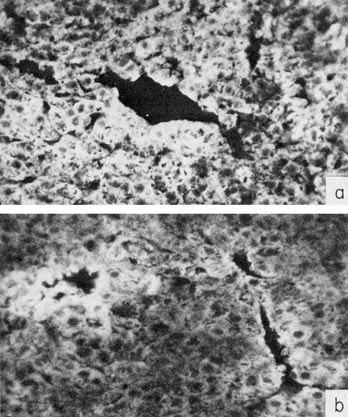
Fig. 7. Immunofluorescence of AFP in the liver of mice during early postnatal life. Sections are treated by rabbit antibody to AFP and then by fluorescein-conjugated antibody to rabbit γ-globulin. X40. (a) The liver of a 1-day-old mouse, (b) The liver of 11-day-old mouse.
Next, a no less important question is whether the hepatic AFP synthesis is completely discontinued in the adult organism, or if some background level of the protein is maintained. AFP has never been detected in adult animal and human sera by the agar precipitation technique. The sensitivity of the method is about 1 μg./ml., which constitutes 0.025% of the maximal AFP concentration in fetal blood. More sensitive methods – aggregate-hemagglutination and immunoradioautography (see Section III), with the sensitivity limit of 0.05 fig./ml. – in our experience likewise do not reveal AFP in human and rat sera, which brings its level below 0.001% of the maximal concentration.
However, in preliminary experiments, Rowe (1970b) has found trace amounts of AFP in some sera of normal donors by an immunoradio-autography test. Using a similar technique we6 have demonstrated recently a regular presence of AFP in adult mouse sera, but not in that of rats or humans. The conclusive answer will probably be obtained from radioimmunoassay, but no corresponding studies have been reported up to now.
|
6 In cooperation with Drs. D. Elgort and S. Perova. |
The problem of background level of AFP is important both for understanding the nature of the phenomenon and in order to find out whether the tolerance to this antigen persists in adult animals and people. If AFP is completely absent from the adult organism, the tolerance may be lost. A possible immunologic response to AFP in patients with hepatomas might influence its blood level and must be considered in the evaluation of "seropositive" and "seronegative" hepatomas. No direct experiments have been reported, however, on immunization of adult animals or humans with the homologous AFP. Thus, the problem of tolerance to AFP, obviously important from the practical and theoretical points of view, still remains open.
Summarizing the available evidence on AFP dynamics during the embryonic and postnatal periods, it may be concluded that phenomeno-logical study of the problem is far from being completed. To obtain a fuller picture, the following directions of research seem to be necessary.
1. Detailed investigation of early embryonic sera: determination of time of AFP appearance relative to periods of functioning of the yolk sac and liver. Comparative studies on AFP from "albumin-less" sera and from sera of adult animals with regenerating liver.
2. Investigation of correlations between AFP production and other functions of the embryonic liver, particularly with the hepatic hematopoiesis.
3. Immunofluorescence study of the yolk sac and liver in ontogenesis, aimed at determining their contribution in the serum protein production during the early and later stages of embryonic development.
4. Analysis by immunofluorescence of the cellular basis for the synthesis of AFP and "adult" proteins: determination of mono- or multi-potency of the hepatocytes in the synthesis of different serum proteins, and of dynamics of the population of AFP-forming cells during ontogenesis.
5. Detection of a possible background level of AFP and of tolerance to it in the adult organism.
In the first study on AFP synthesis by mouse hepatomas (Abelev et al., 1963a,b) it was established that a limited temporary "outbreak" of lFP synthesis occurred in normal adult animals after partial hepatectomy. These observations were repeatedly confirmed later, and the most efficient and reproducible method of AFP induction in mice was shown to be liver regeneration following inhalation of CCl4 vapor (Bakirov, 1968). The response in mice was rather uniform with relatively high secretion of AFP into the blood. AFP appeared 2 days after the animals were poisoned reached a maximum (about 1% of the fetal blood level) on the third day, and then disappeared from the circulation in the course of 8 to 10 days. Laparotomy, inhalation of ether, and other "nonspecific" treatments did not result in resumption of AFP synthesis.
In rats with regenerating liver, an outwardly different picture was recorded. With adult animals, neither partial hepatectomy nor CCl4 vapor inhalation resulted in AFP detectable by the agar-gel precipitation method (Grabar et at, 1967; Stanislawski-Birencwajg et al., 1967; Perova and Abelev, 1967; de Nechaud and Uriel, 1971).
At the age of 5 weeks, "induction" was observed only in some animals after partial hepatectomy (Perova and Abelev, 1967) or administration of CCl4 (de Nechaud and Uriel, 1971). The distinctions between mice and rats could be either quantitative or qualitative, and in order to decide on this point, sera of partially hepatectomized rats were analyzed for AFP by the indirect immunoradioautography test. The result was regular detection of AFP on the second day after the operation with gradual decrease by the ninth to twelfth day (Table II) (Perova et at, 1971).
TABLE II
Alpha-Fetoprotein in Sera of Partially Hepatectomized Ratsa
(Immunoradioautography)
Thus, the dynamics of AFP in rats with regenerating liver has recapitulated that of hepatectomized or CCl4-treated mice, although the absolute values were 10 to 30 times lower.
The experiments allowed one to think that AFP synthesis during regeneration of the liver occurs with general regularity.
Whether AFP appears during regeneration of the liver in humans was of interest not only from the point of view of the scope of the phenomenon, but as a possible source of complications in the diagnosis of hepatic tumors. Immunoprecipitation in agar gel, as a rule, did not reveal AFP in sera of patients with acute hepatitis and cirrhosis, the diseases commonly accompanied by more or less active liver regeneration (see Section VII). It was not until recently that AFP was reported, with this method, in 3 of 375 examined cases of Botkin disease and in 2 of 324 patients suffering from cirrhosis (Tatarinov, 1970; Massiukevitch, 1970) .¹ The AFP concentration was just above the threshold level and the appearance was of short duration. In view of the above instance with rats, the matter had to be reinvestigated with more sensitive methods of AFP detection. No materials were available to us from patients with mechanical injury of the liver, the closest analogy of hepatectomy. But with other liver diseases, the investigation gave quite definite results, when indirect immunoradioautography was used (Table III) (Abelev, 1971; Abelev et al., 1971). Infectious and serum hepatitis were prominent among the liver diseases: in ≈13% of the patients the test revealed AFP in concentrations from 0.05 to 0.5 μg./ml.; 2 patients from the total number of 176 possessed AFP levels considerably higher than the sensitivity limit of agar-gel precipitation.
|
¹ One case of AFP appearance with acute hepatitis and one with cirrhosis were reported earlier by Alpert et al. (1968). See also a case described by Geffroy et al. |
TABLE III
Alpha-Fetoprotein in Patients with Liver Diseases
(Immunoradioautography) a
Sixteen of the patients with AFP in their blood had been followed during the course of disease (Table IV). In all the cases, AFP disappeared from the serum after convalescence. Some of the patients were examined at 7- to 10-day intervals. The results suggested that in viral hepatitis the appearance and disappearance of AFP occurred within a period of 2 months or less. However, the available data are not sufficient for a final conclusion on this point.
TABLE IV
Alpha-Fetoprotein Dynamics in Acute Viral Hepatitis
as Revealed by Immunoradioautography Testa
The suggestion about liver regeneration as a cause of AFP formation in viral hepatitis is founded on principal similarity of the AFP dynamics during this disease and that of mice and rats with regenerating liver. No direct evidence is available, however, which could be obtained, for instance, from detailed comparison of the clinical course with the AFP dynamics. The need in such studies is obvious. There is no doubt, also, of the necessity to compare regenerative processes in the liver of patients who possess or lack AFP in their blood, although such studies in man are not easy to perform.
The patient group with cirrhosis has been sufficiently large, and the fully negative result is unexpected. To explain the contradiction between the absence of AFP in cirrhosis and its presence in infectious hepatitis – both diseases being attended by regeneration of the liver – the following suggestions can be offered: first, that a definite minimal regeneration rate is necessary for AFP "induction" to occur and, second, that the ability to synthesize AFP might become "exhausted" during long-lasting regener ation or with successive cycles of regeneration. The suggestions can be tested readily in experimental models. In this respect, of great interest is the experimental cirrhosis in rats induced by CCl4 (Reuber and Glover, 1970), in which case AFP could be followed during all stages of disease. Repeated induction of the regenerative process in mice by CCl4 vapor may also serve as a good model. Based on the above suggestions, it may be expected, also, that a low level of AFP will be found eventually in patients with active cirrhosis of the liver, especially during the acute stage.
Thus, it may be considered proved that regeneration of the liver results in temporary AFP synthesis in mice and rats, and seems to do so in man. The level of synthesis varies within broad limits in different species. Diagnostic implications of the phenomenon will be discussed in Section VIII.
The early negative findings encountered in detection of AFP during regeneration of the liver in rats and in clinical material have lessened interest in and even cast doubt on the correlation as such. Hence, the "induction" of AFP synthesis under these conditions has received undeservedly little study. The mechanism of "induction" is not known, although the system is probably most suitable for studying this aspect of the phenomenon as a whole. Indeed, AFP synthesis in this case can be induced deliberately under strictly controlled conditions and in adult animals. The response is pronounced and develops without undue variation in individual animals.
1. Cellular Aspects of AFP Synthesis
The first question arising in the analysis of AFP "induction" is whether all the hepatocytes resume synthesis or only some liver cells become "activated." Preliminary evidence, obtained by immunofluorescence on regenerating mouse liver, clearly indicates that AFP is observed only in certain cells distributed in small and rare groups.
The cells become detectable on the second day and are most pronounced on the third day after poisoning animals with CCl4, which corresponds to the maximal level of AFP in the blood serum (Engelhardt et al., 1969). The majority of hepatocytes did not contain AFP.
Studies of this kind should also be made with hepatectomized mice, Which contain no zones of necrosis in their liver, confusing the ordinary picture of that organ. It would be particularly significant to find out whether or not "AFP-positive" cells are revealed around central veins, the areas where AFP synthesis seems to persist longest in the postnatal period.
The next major question is whether the "inducible" cells of the liver are preexisting or appear always as a result of division of precursor cells, cambial cells, or differentiated hepatocytes. This problem can be solved, probably, by combining 3H-thymidine autoradiography with im-munofluorescent detection of AFP in the same sections. An occurrence of even a portion of AFP-containing cells which have not undergone division would indicate preexistence of hepatocytes capable of being induced to synthesize AFP. That would mean that AFP "induction" is dependent on some external factors, inducers or inhibitors which activate or switch off the synthesis. On the other hand, if it is found that all the "AFP-positive" cells belong to the proliferative pool of the regenerating liver and appear only as a result of division of precursor cells, the latter can be either the division of a mature hepatocyte, attended by dediffer-entiation, or that of a cambial liver cell, leading to its differentiation into an AFP-producing hepatocyte.
2. Genetic Aspects in the "Induction" of AFP
For elucidating factors involved in AFP "induction" it is of interest to use line distinctions in this character, found in mice of C3HA8 and C57BL/6 strains (Bakirov and Abelev, 1968). The distinctions are of quantitative nature, but quite significant: on the average, the AFP levels in the strains differ by a factor of 10. Hybridological analysis could show the number of genes determining this trait. It seems feasible also to derive coisogenic pairs, different only in AFP formation levels. The availability of such a specific pair, with distinction only in the studied character, would be of undoubted interest for determining factors which control AFP synthesis and understanding species differences in activity of the synthesis during regeneration of the liver.
|
8 The C3HA response is typical for most mouse strains tested. |
In this connection, the existence in mice of a detectable background level of AFP, as distinct from the rat and man, is of primary importance. It seems most likely that the degree of "induction" of AFP during regeneration is in positive correlation with the background level of AFP. A quantitative investigation of this suggestion in "inducible" and "low-inducible" lines and their hybrids might give a definite answer to the question.
The evidence obtained with experimental models and in the clinic may be considered together in view of the principal similarity of the phenomenon in animals and man. This is especially justified since each of the approaches has its advantages: with animal tumors, a more detailed analysis has been done while the clinical material examined has been by far more rich and diverse than that from experimental models. Naturally, one must always bear in mind the species differences as well as the differences in etiology of hepatomas in experiments and nature, which exclude direct transfer of empirical generalizations from experimental model systems to man. First, we shall consider the problem of localization of the AFP synthesis in cancer of the liver, upon which proper evaluation of both clinical and experimental evidence is dependent.
When AFP appears in the blood of a tumor-bearing animal or patient, both the tumor itself and the affected liver could be the source of the embryonic protein. There is sufficient evidence to believe that tumor constitutes the main site of AFP synthesis in the organism of the host, but the possibility of the synthesis being resumed by the liver, even if in some exceptional cases, by no means can be excluded.
Most convincing evidence about the site of AFP synthesis was obtained in experimental model systems. In experiments with a transplantable hepatoma of mice we have demonstrated that the tumor continues to synthesize murine AFP following its heterotransplantation into cortisone-treated rats or explantation in vitro (Abelev et al., 1963a,b). Subsequent studies with stable cultures of the same tumor showed that, at least during first generations they maintained the ability to synthesize AFP in vitro together with albumin and transferrin (Irlin et al., 1966). Accumulation of AFP was observed also in primary cultures of Zaidela's hepatoma of the rat (Perova and Abelev, 1967). Experiments of Hull et al. (1969b,c) on cultivation of primary hepatomas of monkeys, induced by carcinogen, have showed that AFP, albumin, and transferrin are synthesized by them in vitro.
Thus, data obtained with hepatomas of mice, rats, and monkeys convincingly demonstrate AFP production by tumors themselves. It should be emphasized that the evidence in itself does not at all exclude possible participation of the liver in the production.
Naturally, it is a more difficult task to determine the site of AFP synthesis in humans suffering from liver cancer. Three groups of evidence testify in favor of AFP production by tumor itself, apart from the analogy with the situation in animals: the highly specific correlation of AFP with hepatocellular cancer, the disappearance of AFP after surgical removal of the tumor, and the detection of AFP in tumor cells by immunofluorescence. The first group of evidence will be especially considered in the next section, while two others are discussed here.
Surgery with hepatomas is extremely rare. We have observed only one case. In a patient who had a high blood level of AFP, there was a sharp decrease in AFP, at times its complete disappearance, when the liver lobe affected by tumor had been ablated (Morkhov and Sokolov, 1970).
More information on the AFP synthesis site in human cancer of the liver has been obtained recently by immunofluorescence. It has been mentioned that AFP can be readily localized in tissues by the technique of Sainte-Marie. A major complication in work with hepatomas, especially with the autopsy material from humans, is connected with secondary nonspecific uptake of AFP from the serum by dead cells and, in general, by cells with impaired permeability. These are necrotic cells in tumor and dead or dying cells in normal tissue, because, usually, the material is available only several (2-6) hours after clinical death. To differentiate AFP-forming cells from those passively acquiring it along with other proteins of the plasma, we used a special control: parallel examination of the same cells in serial sections for presence of human γ-globulin (Engelhardt et al., 1971).
γ-Globulin is known to be elaborated only by plasma cells and does not penetrate living cells of the liver or tumor, while penetrating freely into dead cells. Parallel study of the same areas in sections of the liver or tumor for presence in the cells of AFP and human γ-globulin, has indeed showed the existence for both antigens of completely coinciding areas, with identical localization, as well as of tissue regions where only AFP was localized. A typical appearance of the "overlapping" and "nonoverlapping" distribution is shown in Fig. 8. Specific localization of AFP has been observed only in tumor tissue. The liver, lung, and kidney of the patient exhibited no AFP distribution differing from that of γ-globulin (Gusev et al., 1971b).
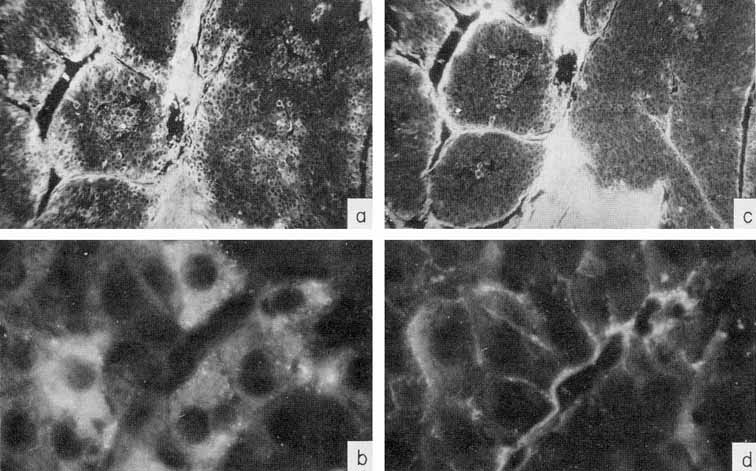
Fig. 8. Localization of AFP and γ-globulin in a human hepatocellular carcinoma, (a-b) A tumor section treated by rabbit antibody to AFP and then by fluorescein-conjugated antibody to rabbit γ-globulin. X10 and X90, respectively, (e-d) A parallel section treated by rabbit antibody to human γ-globulin and then by fluorescein-conjugated antibody to rabbit γ-globulin. X10 and X90, respectively. Note the γ-globulin-free cells containing AFP. (According to Engelhardt et al., 1971.)
The immunofluorescence evidence is quite convincing. But the conclusive proof of AFP synthesis by human hepatocellular tumors could be provided by evidence on AFP synthesis by cultured hepatomas. It should be supposed that such evidence will soon be available.
Thus, the combined findings from experimental model systems and clinical material indicate that AFP synthesis is resumed by cells of hepatocellular carcinomas themselves. On the other hand, the occurrence of AFP synthesis during regeneration of the liver must be taken into account as ah additional possibility weakening the correlation of AFP with cancer of the liver.
This section will be devoted to results of experimental and clinical studies on detection of AFP in cancer of the liver, other tumors, and noncancerous diseases of the liver. No consideration is given to AFP in embryonic tumors, since the problem requires special analysis, presented in Section IX.
Tables V and VI summarize published results on liver tumors in experimental animals together with appropriate control groups. Table VII presents clinical data available to us on more than 1000 cases of primary cancer of the liver and several thousand disease cases in the control group. It must be emphasized that the antisera used by different workers for AFP determination in humans, in the majority of cases, had been collated. All data presented in Tables V to VII were obtained by agar-gel precipitation.
TABLE V
Alpha-Fetoprotein in Animal Hepatomas, According to the
Agar-Gel Precipitation Test
TABLE VI
Alpha-Fetoprotein in Nonhepatic Tumors and Nonneoplastic Conditions
It should be kept in mind that the total number of hepatoma patients in Table VII may be somewhat overestimated, because some authors included the same data in several publications. In known instances, the corresponding data in Table VII have been marked. From the reported results a few quite significant general conclusions can be drawn.
1. The appearance of AFP in the blood of adult animals and humans is highly specific for hepatocellular cancer and for mixed hepato- and cholangiocellular cancer of the liver. On the average, AFP has been found in about 70% of histologically confirmed hepatocellular cancers in humans.
2. The cholangiocellular cancer of the liver is not associated with AFP production.
3. Metastatic tumors of the liver, as a rule, are not associated with appearance of AFP, nor are malignant tumors of nonhepatic origin.
4. Liver diseases of noncancerous nature are usually not accompanied by AFP formation.
5. There is a significant group of histologically confirmed hepatocellular carcinomas in which AFP has not been detected (about 30% in humans).
6. A small portion of clinical cases constitute a histologically confirmed group of "false positives."
The material presented in the tables seems to be quite sufficient for a general conclusion about the practical value of the AFP test for differential diagnosis of hepatocellular cancer.
However, both the analysis of the nature of the phenomenon and further improvement of the test require additional evidence, primarily from studies of the differences between "AFP-positive" ("AFP+") and "AFP-negative" ("AFP–") hepatomas and of cases in which AFP appears in the absence of hepatocellular cancer.
TABLE VII
Alpha-Fetoprotein in Hepatocellular Cancer and Other Diseases of Man,
According to Gel Precipitation Method a
TABLE VII (continued)
TABLE VII (continued)
The first problem which should be considered in comparing "AFP+" and "AFP–" hepatomas is whether they have qualitative or only quantitative differences between them. The corresponding data are discussed below.
In connection with data considered in the previous section, it has been mentioned that all of them were obtained by a method possessing a definite sensitivity limit which, in the end, is of arbitrary value. Until quite recently, we did not know events occurring in the "subthreshold" area, or the degree in which the specificity of the phenomenon and, respectively, of the diagnostic test is maintained, when more sensitive methods of AFP detection are employed.
Blood levels of AFP with hepatomas in animals and man vary within very broad limits, in man no less than 1000-fold, from 1 or 3 μg./ml. 9 up to 5 or 7 mg./ml. (Masseyeff et al., 1968; Purves et al., 1968, 1970). There is no doubt that some "AFP–" hepatomas elaborate subthreshold quantities of AFP and would be in the "AFP+" group with highly sensitive methods.
|
9 Sensitivity threshold of immunodiffusion methods. |
Some impression of the size of the "subthreshold" group of hepatomas can be gained from the analysis of hepatoma distribution curves based on their AFP production. By extrapolation of the curves beyond the sensitivity limit of the immunodiffusion method, the theoretically expected gain for the group can be obtained. Unfortunately, quantitative data are not given in the overwhelming majority of reports. Besides, the distribution picture is distorted by cases of erroneous diagnosis which, naturally, accumulate in the "AFP–" group.
The distribution curves have been presented in only two publications: by Purves et al. (1968, 1970c) for the Union of South Africa and Masseyeff et al. (1968) for patients from Senegal. According to Purves, extrapolation of the distribution curve below the sensitivity limit of the agar-gel precipitation method, indeed, suggests existence of "AFP+" hepatomas in that range. As their preliminary results indicate with the radioimmunoassay, about one-third of "AFP–" cases are transferred into the "AFP+" group, resulting in about 90% of "AFP+" hepatomas, on the whole, for Bantu of South Africa (Purves et al., 1970c).
The distribution curve for Senegal (Masseyeff et al., 1968) is rather peculiar: it has a distinct maximum in the range of ≈50 μg./ml. with symmetric slopes in both directions. But, near the sensitivity limit of the method, there is a noticeable increase, so that it is difficult to predict the ultimate direction of the curve (Fig. 9a). Of similar appearance is the distribution curve plotted using our data with the International Experiment materials for seven countries (O'Conor et al., 1970). Again, there is a maximum in the range of 50 to 70 μg./ml., slopes on both sides, and a rise in the region of threshold values (Fig. 9b).
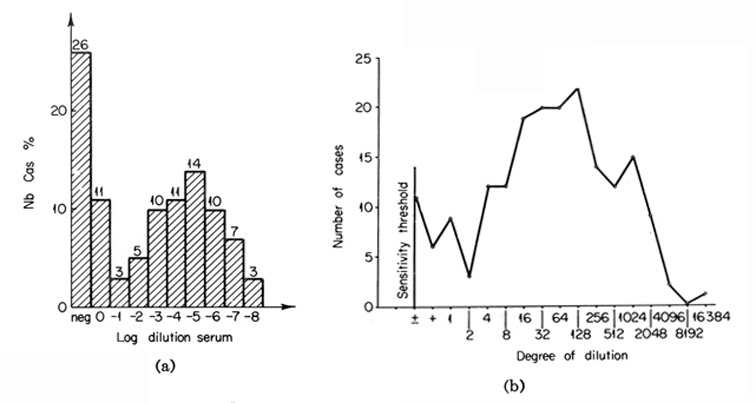
Fig. 9. The distribution of serum AFP levels in patients with cancer of the liver. (a) The distribution curve for Senegal. (From Masseyeff et al., 1968.) (b) The istribution curve for seven countries, which includes all "AFP+" cases. (According to O'Conor et al, 1970.)
It appears therefore, that although the distribution curves clearly suggest the possibility of occurrence of hepatocellular cancers with subthreshold levels of AFP, it may not be decided from their appearance, whether the particular range contains a "fading" branch of the curve or a new maximum.
The above-mentioned distribution curves are related to countries where a high percentage of "AFP+" hepatomas is observed. It would be very important to have similar curves for those countries which have considerably lower incidence of such tumors. It is not impossible that the corresponding curves would be "cut off" by the sensitivity limit of the method.
Experimental analysis of the quantitative aspects of AFP production by hepatomas has just been started. According to our data (Abelev, 1971, Abelev et al., 1971), the indirect immunoradioautography test has revealed 22 positive cases in a group of 48 "AFP–" hepatomas studied (46%). The examined collection included 36 sera from Dakar10 and 12 from the USSR. The percentage of "AFP+" findings in the former group was considerably lower (37%) than in the latter (75%). The material was not large and not homogeneous with respect to diagnosis. It is quite possible that the disagreement between the groups was due to unequal accuracy of diagnosis. It is only natural that cases of misdiagnosis would accumulate in the "AFP–" group, bringing down the relative gain in "AFP+" cases. For the most part, the Moscow collection (11 cases) had histological confirmation while that from Dakar was confirmed in only half of the cases (Masseyeff et al., 1968).
|
10 Courtesy of Prof. Masseyeff and Dr. Leblanc (Dakar University, Senegal). |
The available material is not sufficient for definite conclusions, but it clearly indicates the prospects for employing highly sensitive AFP determination tests for further progress in the diagnosis of liver cancer. Such development entails, however, the inevitable lowering of the specificity of the AFP test. This point is additionally discussed in Section VIII.
The pronounced increase in the "AFP+" group with higher sensitivity of the detection method also permits the suggestion that two kinds of hepatoma cases may exist, one corresponding to the maximum of 50 to 70 (g. AFP/ml. in the distribution curve, and the other to a maximum in the subthreshold range. It may be that the different incidence of "AFP+" hepatomas in various countries is due only to different occurrence of these two kinds of cases. This hypothesis requires quantitative determinations of AFP by usual and highly sensitive methods in groups of patients from countries with high and low incidence of "AFP+" hepatocarcinomas, as well as quantitative studies on experimental "models."
In order to get a deeper insight into the mechanism by which the AFP synthesis is resumed in hepatomas, as well as for practical purposes, it is necessary to know conditions under which the phenomenon takes place and correlations, if any, of this trait with other characters of hepatomas. It seems more accurate in this respect to speak of correlations between the tumor characters and the level of AFP synthesis, but until the group of "AFP–" hepatomas is sufficiently well analyzed, we have to deal with "AFP+" and "AFP–" groups, bearing in mind the provisional character of this division.
1. The Dependence of the Proportion of "Seropositive" Hepatomas on Areas of the World
In analyzing the frequency of occurrence of "AFP+" hepatomas in various parts of the world, considerable differences have been noted by all investigators engaged in this field. Moreover, as Hull et al. (1970) concluded, there is a positive correlation between the proportion of "AFP+" hepatomas and the incidence of liver cancer in a particular area of the world. Indeed, the highest percentages of "seropositive" hepatomas have been observed for areas endemic for primary cancer of the liver: the South African Union (82%), Senegal (79%), Congo (77%), Mozambique (67%), Indonesia (87%). The proportion of "seropositive" hepatomas among the native population in the USA, England, France, and Greece is considerably less – from 50 to 60% – but it approaches maximal values in those who come from Africa. Values for the USSR (Moscow) and Uganda are intermediate (64%). Unexplainably high are the figures for Astrakhan (96%) and Siberia (82%) (see Table VII).
Of course, not all of these data are equally comparable since they differ by exactness of diagnosis (the proportion of clinically and histologically confirmed cases in each of the groups), by selection of patients (a tendency to follow each "AFP+" case and loss of some "AFP–" cases), and by modifications of the AFP test used by different laboratories. But, even with all these reservations taken into account, the regularity seems to be evident: the incidence of "AFP+" hepatomas is higher in areas endemic for hepatocellular cancer.
This conclusion raises a number of problems, including those of dependence of the "serological type" of the tumor on the etiological agent, chemical nature of carcinogen, dose and route by which it penetrates the organism, on genetic and physiologic peculiarities of a particular population, and on characteristics of the tumors arising in different populations.
2. Dependence on Carcinogen
Among the hepatomas of rats, mice, and monkeys which are characterized in Table V above, there are tumors induced by various carcinogens – OAAT, 3'-Me-DAB, DAB, nitrosoamine and anatoxin, as well as spontaneous hepatomas and preneoplastic nodules resulting from pyridoxine deficiency. As can be seen, "AFP+" tumors are present in all groups of hepatomas, both spontaneous and induced, with one notable exception. Aflatoxin, a most probable carcinogen for man, has induced hepatomas in rats, which formed no AFP. It seems to us that these observations indicate the need of further studies. They include results of two series of experiments with primarily induced tumors, which malignancy has not been proved by transplantation. For definite conclusions, it is necessary to study transplantable alfatoxin-induced hepatomas with application, if required, of highly sensitive tests for AFP detection.
In the rest of the groups, no correlations can be found between the carcinogen used, on the one hand, and the frequency of "AFP+" hepatomas, or the AFP level, on the other. Moreover, one carcinogen (3'-Me-DAB) acting on the same animals under similar conditions of application exhibited rather significantly differing results: 52% and 100% of "AFP+" hepatomas (Stanislawski-Birencwajg et al., 1967). It has been noted in all studies that with hepatomas induced by the same carcinogen there are great differences in the levels of AFP.
In spite of the fact that the above material is small, episodic, not always studied under comparable conditions, and clearly requires confirmation, we are inclined to believe that AFP production is determined rather by the character of the induced tumor than by etiologic factors.
3. The Dependence of AFP Production on Biological Characteristics of the Tumor
In our initial study on AFP formation by mouse hepatomas, it was noted that the degree of malignancy positively correlated with the level of AFP production by the tumors (Abelev, 1965a, 1968). This is shown in Table VIII together with more recent data which have supported and extended the observation. As can be seen, trace amounts of AFP, detectable only by the immunoradioautography test were associated with growth of hepatomas, which type may be defined as "minimally deviated from normal." Threshold levels (by agar-gel precipitation) were observed with slowly growing strains while three fast-growing anaplastic hepatomas produced the greatest amounts of AFP. Similar correlation between AFP formation and degree of malignancy was noted also when primary OAAT-induced hepatomas were compared with their first generations and transplantable stable strains (Abelev, 1965a, 1968; Khramkova and Guelstein, 1965; Guelstein and Khramkova, 1965).
TABLE VIII
Alpha-Fetoprotein in Mice Bearing Hepatomas of Different Growth Rate
The above observations and conclusions strongly disagree with those of other authors who studied primary tumors in rats, monkeys, and man. Thus, no regularity was found when AFP production was analyzed with respect to histological type of primary tumors of rats (Stanislawski-Birencwajg et al., 1967) and monkeys (Hull et al., 1969b,c).
Also unsuccessful were attempts to reveal any correlations for human tumors between the occurrence or level of AFP in the blood and the degree of malignancy or histological type of the tumor. In all groups analyzed in this respect no correlation was found (Masseyeff et al, 1968; Abelev et al, 1967a; Purves et al, 1970c; Foli et al, 1969; O'Conor et al, 1970). Only in the study of Purves et al. (1970c) was a slight tendency noted: the percentage of "AFP+" hepatomas was lower in patients with highly differentiated tumors than in those with less differentiated ones, and became lower again in a group with anaplastic cancer of the liver.
It is difficult at present to find explanations for the existing contradictions. It is possible that the regularity noted in mice is an occasional observation, which will not be corroborated with larger material. It seems to us, however, that the reason for the discrepancy may lie with the fact that tumors studied in the former case were mostly transplantable, while those in the latter case were primary. It is natural to expect that any correlations between AFP production and some other character of the tumor should be more pronounced in comparing relatively homogeneous transplantable tumors which have undergone extended selection. Primary tumors, especially in man, are large and heterogeneous. Individual nodules may differ in the degree of malignancy and differentiation. To look for a correlation there, obviously, is more difficult.
It seems to us that the problem can be adequately solved by measuring AFP in a series of transplantable Morris hepatomas of rats which present a complete spectrum of tumors discretely differing in the degree of malignancy, as well as in separate biological and biochemical characters (Morris et al., 1964).
It is necessary, also, to elaborate quantitative criteria for the evaluation of AFP production by an individual tumor, perhaps on the basis of incorporation of 14C-amino acids in this protein in vivo, taking into account the weight of the tumor. It is also extremely desirable in this type of study to use the high-sensitivity tests.
As for primary tumors, especially those of man, another approach seems to be promising: a study of specific features of AFP-producing cells and of regularities determining their appearance within tumors of various structure. The method of detecting AFP by immunofluorescence (see Section VII,A) provides opportunities for such investigations. Initial results obtained with this method in human hepatomas indicate that only few cells of the tumor specifically contain AFP, and that these cells always form groups "gravitating" to vessels in the tumor (Gusev et al, 1971b). The latter evidence strongly suggests that the resumption of AFP synthesis in cells of the tumor is probably not a random process, and that there is some regularity in appearance of such cells, which can be revealed, possibly, by combining methods of immunofluorescence, histochemistry, and autoradiography.
Demonstration in human and animal hepatomas of the isozyme spectrum peculiar to the embryonic liver (Schapira et al., 1963, 1970; Nordmann and Schapira, 1967; Uriel, 1969) is of undoubted interest for determining correlations between AFP production and other biochemical characters of hepatomas. It is especially important that the shift toward embryonic type is differently expressed in individual human tumors and in various strains of transplantable hepatomas of the rat. In the latter case, aldolases of the embryonic type were detected only in fast-growing tumors, whereas slowly growing tumors possessed the isozyme typical of the adult liver (Schapira et al., 1970).
Parallel investigations of AFP formation by hepatomas, possessing the adult or embryonic aldolase spectra, would be of great interest. Also important would be parallel immunofluorescence studies on localization of AFP and embryonic type aldolase in the tumor tissue, especially with human primary tumors.
4. The Dependence of AFP Synthesis on Stage of Cancerogenesis and Tumor Progression
This aspect is important both for understanding the nature of the phenomenon and the evaluation of feasibility of early diagnosis of hepatomas. Practically no experiments have been made in this direction in simple mouse and rat "models," but there are studies in monkeys and single observations in the clinic. Foy et al. (1966), working with baboons deprived of pyridoxine in their diet, have shown that such animals develop lesions in their liver which resemble hepatocellular carcinoma in structure.
The lesions were not malignant tumors, but are considered by the authors as preneoplastic formations. Monkeys fed pyridoxine-less diets contained AFP in their blood, its level being not transitory, but maintained in the course of several months (Foy et al., 1970a,b). These observations indicate the possibility of AFP appearance in monkeys during the process of cancerogenesis.
Investigations of Hull et al. (1969b,c) on monkey hepatomas induced by nitrosoamine showed that AFP may appear in the blood before the tumor is detectable by palpation and before any clinical signs appear. Besides, the authors noted that a high level of AFP can be observed with a very small tumor. These observations, although not fixing a definite stage in cancerogenesis, clearly show that AFP in AFP- producing tumors is detected during early clinical stages of development of the tumor.
Clinical evidence which bears on this problem is episodic. It is of special interest, for determining the clinical stage at which AFP may appear, to examine cases for which the initial time of AFP detection has been registered. We have had only two observations of this kind among studied patients (Khasanov et al., 1971). Patient S., suffering from cirrhosis and severe pancreatitis, gave a negative AFP test. Two months after the first examination a weakly positive reaction for AFP developed. No clinical symptoms of primary cancer of the liver were observed. Following AFP detection, the patient was twice examined by scanning of the liver and selective angiography, but it was not possible to localize exactly the suspected tumor. During the following months, the AFP level increased continuously. In 7 months after the initial detection of AFP, some increase in dimensions of the left liver lobe was noted. Clinical signs of progressing liver cancer became evident only 1 year after the appearance of AFP. The lethal outcome from another cause confirmed the presence of primary cancer of the liver. This case clearly shows that with "seropositive" hepatomas AFP may appear at sufficiently early stages of disease, prior to its clinical symptoms. But there is no doubt that opposite cases may exist, when AFP appears after the clinical symptoms (Hull et al., 1969a; Khasanov et al., 1971).
Obviously, the problem cannot be solved empirically, and thorough experimental analysis is required. Studies on chemically induced can-cerogenesis in mice and rats, using highly sensitive tests for AFP detection and immunofluorescence, seem to be quite expedient.
5. The Dependence of the Incidence of "Seropositive" Hepatomas on Age of the Patients
This problem has not been studied at all in experimental models. In the analysis of a large amount of material from Senegal (Masseyeff, 1971), a slight tendency was revealed toward lower percentage of "AFP+" hepatomas with increasing age of the patients. However, it should be noted that the material examined was from Africa, where a relatively young population group is affected by cancer of the liver, and where the percentage of "AFP+" hepatomas is rather high (≈75%). A greater incidence of "AFP+" hepatomas in males has also been noted (O'Conor et al, 1970).
Mawas et al. (1970, 1971) demonstrated a definite correlation between the age and the incidence of "AFP+" hepatomas in a group of patients from France. In children not older than 11 years of age, ≈90% of hepatomas (21 cases of 24) appeared to be "AFP+", which greatly exceeds the average incidence of such hepatomas for France (60%), and even the corresponding indexes for countries of Africa and Southeast Asia.
The work of Mawas and his associates has been corroborated by Bagshawe and Parker (1970) on a group of patients from Kenya. Nine patients with hepatocellular cancer, whose age was from 10 to 30 years, were 100% "AFP+"; in the patient group aged 31 to 40 years, the proportion of "AFP+" cases was 66% (4 cases out of 6), while at ages over 40 years there were only 22% of "AFP+" patients (2 out of 9). These extremely remarkable observations could throw light on the reason why there are geographical differences in the incidence of "AFP+" hepatomas. It is well known that the age of hepatoma patients from Africa is, on the average, some three decades less than that of patients from Europe and America.
The problem of dependence of the incidence of "AFP+" cases upon the age merits a most thorough investigation and accumulation of greater material. This may help to elucidate whether hepatomas developing at a young age possess any specific features, or whether there is some other determining factor which is extraneous to the tumor and changes with the age of the organism.
The age dependence strongly suggests the existence of an external factor ("inducer"), which influences the rate of AFP synthesis in tumor tissue, and which diminishes with age. It may be supposed that a certain part of hepatomas, belonging to the "subthreshold" group according to their AFP formation, are competent to respond to the "inducer" by elevated AFP production, and that the same phenomenon underlies AFP "induction" during regeneration of the liver. If it is so, we can expect that in younger patients, possessing a greater level of the "inducer" than the older group, an increase in AFP production by low-productive ("AFP–" in the standard test) hepatomas will take place and that they will appear in the "AFP+" group.
This supposition can be tested experimentally. First, there must be a deviation from the symmetric shape in patient distribution curves, based on AFP level, in the group of young patients, as compared with elderly patients, with an increase in the range of low AFP concentrations.
Second, according to the above suggestion, an exposure to higher concentration of the "inducer" should result in increased AFP synthesis on the part of highly differentiated mouse hepatomas which produce little AFP. Higher levels of the "inducer" may be expected either in the early postnatal period or during regeneration of the liver. A tumor growing in such conditions should produce more AFP than that in normal adult mice. This can be checked, for example, by the immunofluorescence method, which allows one to calculate "AFP+" cells directly in the tissue studied.
Age dependence may alternatively be explained from quite a different position. If it is assumed that tolerance to AFP in humans is lost with age, then it will follow that AFP elimination from the blood would take place in adult patients much more frequently than in younger patients, creating a seeming impression of dependence between the hepatoma "serotype" and the age of the host.
Thus, in the consideration of possible correlations between AFP production and etiologic and pathogenic factors, a number of remarkable regularities and contradictions are revealed. This character of hepatomas has been shown to depend on "endemicity" of the area and age of the patients. The evidence on AFP production as related to degree of malignancy appears to be contradictory, while the role of the carcinogen and of the stage of cancerogenesis has been studied very little. The available evidence does not permit one to present any general picture concerning the resumption of AFP synthesis in tumors. But there are approaches and still unused opportunities for analyzing the problem.
In this chapter, we should like to discuss the use of the AFP test for differential diagnosis of hepatocellular cancer and for its early diagnosis.
The differential diagnosis based on the standard AFP test may be considered, on the whole, a solved problem. The summary of the corresponding studies, presented in Table VII, testifies for very high specificity of the AFP test when working with the agar-gel precipitation methods.
It should be noted that the percentage of "AFP+" cases was, as a rule, significantly higher in the groups with histologically confirmed hepatocellular cancer in comparison with those which include both types of diagnosis. This indicates that the AFP test give less false positive results than diagnoses based on clinical data only.
False positive reactions can also be observed here, most likely with cases of infectious and serum hepatitis and of stomach cancer metastatic to the liver. In the former cases, to obtain a differential diagnosis, the reversible character of AFP dynamics with noncancerous diseases of the liver may be taken into account, besides the clinical symptoms. Repetition of the analysis in the course of 2-4 weeks will indicate whether the production of AFP is transient or an increase in its level takes place.
In case of metastases to the liver, the situation is more complicated, as the reason for AFP production is not clear. Tumor itself is unlikely to be the source of AFP. More probable is another mechanism, namely, via necrosis and regeneration of the liver parenchyma.11 In this case we also have a right to expect transient appearance of AFP and its very low levels.
|
11 For instance: liver regeneration due to biliary obstruction (Cameron, 1935; Fahan et al, 1966). |
Another, more "ponderable" problem is that of "false negative" reactions, especially for America and Europe where they amount to 50% of hepatoma cases. We consider it quite substantiated here to use highly sensitive tests, first of all the immunoradioautography. In our experience, 75% of "false-negative" hepatomas from the USSR (9 out of 12 cases) appeared to be clearly positive with this test. The reaction itself, being absolutely specific for AFP, is not much more difficult to perform than the agar-gel precipitation test and thus is quite feasible in hospital laboratories.
However, when dealing with AFP levels of the order of 0.05 to 0.5 (g./ml., much more frequent are "false positive" reactions due to pregnancy, viral hepatitis, metastases to the liver and other liver diseases (Table IX).
TABLE IX
Alpha-Fetoprotein in Nonhepatoma Patients According to
Immunoradioautography Test a
Thus, using the immunoradioautography test, we may expect to have about 90% of serologically revealed hepatomas (together with those diagnosed by agar-gel precipitation) and about 15% of "false positives" in groups with metastatic liver cancer and viral hepatitis. As was already mentioned above, the basis for differential diagnosis in case of Botkin disease can be obtained by following the AFP dynamics, together with clinical symptoms (Table IV). It is well established that with primary cancer of the liver there is either a stable or a slowly growing AFP level (Purves et al., 1970c; Masseyeff et al., 1968). Detailed studies on AFP dynamics in metastatic liver cancer are urgently required.
Can the AFP test provide any additional information on the clinical state of a patient? Thorough investigations in this direction, carried out by Masseyeff et al. (1968) and Purves et al. (1970c) on large groups of patients in Senegal and South Africa, permit only a negative answer to this question. No correlation is apparent between the AFP level in a patient and the stage or gravity of the disease. Observation of AFP levels may serve, apparently, as a control of success of surgical treatment, judging from disappearance of AFP after resection of the tumor, or an indication of a relapse or metastases if AFP reappears.
As far as early diagnosis of hepatocellular cancer is concerned, we have already discussed what evidence there is on the time of AFP appearance during development of liver cancer. Experiments in monkeys and single observations in the clinic permit an optimistic view of prospects of an early diagnosis in some cases of hepatocellullar cancer. But a direct answer may be obtained only from broad epidemiological examination of the population in areas of the world endemic for liver cancer.
Such investigations, employing the standard AFP test, were being conducted by the International Agency for Research on Cancer in the Ivory Coast, at the time the present review was written (Annual Report of International Agency for Research on Cancer, 1969). It is very tempting to introduce highly sensitive tests in studies of this kind. In spite of the inevitable increase in the percentage of false positive reactions, these methods provide a considerably better chance of detecting clinically unapparent forms of liver cancer. It is essential for such work to follow AFP dynamics in detected positive cases.
Broad examinations of certain population groups by the high-sensitivity tests have some technical difficulties. To perform the radioimmunoassay or immunoradioautography with samples from tens of thousand people is a complicated, time-consuming, and expensive undertaking. Here, it may be extremely helpful to apply the aggregate-hemagglutination reaction.
In a comparative trial of the aggregate-hemagglutination reaction and immunoradioautography, performed on the same collection of sera, it has been found that the former reaction gives considerably more positive results than the latter, mostly with low dilutions of sera (1:8 and 1:16) (Abelev et al., 1971). This disagreement was not due to sensitivity of the methods, which was comparable.
It was found recently that the disagreement between two tests could be sharply diminished, if not completely excluded, by the use of additional control – erythrocytes sensitized by nonimmune serum. The "false positive" samples were equally active in agglutination of both types of erythrocytes – conjugated with immune as well as with normal serum. The absorption of such serum samples by erythrocytes loaded with nonimmune serum inhibited their reactivity against the control erythrocytes. The same treatment did not influence the specific activity of AFP-containing samples.
According to our data, however, the aggregate-hemagglutination test gives almost no "false negative" reactions, i.e., it has not, practically, been negative with samples obviously positive for AFP in the immunoradioautography test. The only group in which such results were registered was that of pregnant women (76% positives by the aggregate-hemagglutination reaction and 98% positives according to the immunoradioautography test). No false negative reactions were observed in patient groups with primary liver cancer, embryonal tumors, infectious hepatitis, etc., provided the aggregate-hemagglutination titer of a serum equal to 1:8 was recorded as a positive reaction.
It is clear, therefore, that the aggregate-hemagglutination test can be used for preliminary screening, with subsequent verification of selected sera by the immunoradioautography test. The simplicity and speed of performance of the aggregate-hemagglutination reaction allows its application in broad epidemiological surveys. One operator is capable of testing several hundred sera a day.
Summarizing the discussion on the clinical aspects of the phenomenon, the following three statements can be made.
1. The differential diagnosis of hepatocellular cancer on the basis of the AFP test, carried out by the agar-gel precipitation method, is highly specific, but always gives a definite percentage of false negative results.
2. The proportion of diagnosed hepatomas can be significantly increased with application of highly sensitive tests for detecting AFP. False positives may be obtained in pregnant women and a part of patients with metastases to the liver and viral hepatitis. To obtain a differential diagnosis in the above cases, it is necessary to follow the AFP level in dynamics.
3. For the prospect of an early diagnosis of liver cancer, it is expedient to undertake a broad-scale study of the population in the "endemic" areas, with application of highly sensitive tests for AFP detection and regularly following the dynamics of positive reactions.
This problem has been much less studied than that of AFP with liver cancer, although its practical importance for countries of Europe and America is, probably, not less than in liver cancer. First of all, it should be emphasized that studies in experimental models are completely lacking in this case, which, naturally, deprives the problem of a basis for theoretical exploration. This is the more a pity, as tetrato-carcinomas of mice are known and well-studied tumors (Kleinschmit and Pierce, 1964; Kahan and Ephrussi, 1970; Rosenthal et al., 1970).
AFP has been reported to occur in blood of patients with malignant teratoblastomas of the testis and ovary, as well as in those rare cases when this tumor has retroperitoneal localization. Published evidence, obtained with the agar-gel precipitation method, is summarized in Table X. It follows from these data that:
1. The occurrence of AFP is specific for malignant testicular and ovarian teratocarcinomas with elements of embryonal cancer.
2. Tumors of this type are associated with AFP in about 50% of cases.
3. Other embryonal tumors – seminomas, chorionepiteliomas, neuro- and nephroblastomas and Wilms' tumors are not accompanied by AFP in patients' blood.
Thus, AFP may be of quite definite significance in the differential diagnosis of the germinal cancers. Besides, in the case of "AFP+" teratoblastomas, the AFP level reflects the effectiveness of surgery and chemotherapy (Mawas et al., 1969a,b).
TABLE X
Alpha-Fetoprotein in Patients with Embryonal Tumors as Revealed by Agar-gel Precipitation Test
An important observation was made by Mawas et al. (1971) who noted a correlation between the incidence of "AFP+" teratoblastomas and the degree of their malignancy. Of 29 cases which they studied, AFP was found in 15, while from the remaining 14 "AFP–" cases, 13 tumors underwent complete regression. It is possible that with tumors of this type, the occurrence and level of AFP may characterize to some extent the character of the tumor and the course of the disease.
It was natural to examine patients with embryonal tumors using methods of higher sensitivity. The corresponding results, obtained with indirect immunoradioautography, are presented in Table XI. Considering these results, it should be borne in mind that only those patients were reexamined in the teratoblastoma group who failed to exhibit AFP in the standard test. The results make evident both a pronounced increase in serologically diagnosed teratoblastomas and some decline in the diagnostic specificity of the reaction. It should be recalled in this connection that teratoblastomas are most frequent in children, and that child hepatopathy is often associated with "false positive" reactions (Masopust et al., 1971). This possibility should be taken into account when highly sensitive tests are applied for the diagnosis of child tumors.
TABLE XI
Alpha-Fetoprotbin in Patients with Embryonal Tumors as Revealed
by the Immunoradioautography Test a
Thus, the AFP test presents undoubted clinical interest with teratoblastomas, and an extension of studies in this direction is extremely desirable.
As far as the theoretical aspect of AFP formation with embryonal tumors is concerned, it remains completely unstudied. There is no adequate proof that AFP is formed by tumor itself, although it is most probable, taking into account the specificity of the phenomenon and the fact that the AFP formation is stopped by resection of the tumor. It cannot be excluded, of course, that teratocarcinomas induce AFP synthesis in the liver. The finding of AFP in the cells of tumor by immunofluorescence technique (Mawas et al., 1969a) is not quite convincing since passive uptake of AFP from the serum was not excluded (see Section VII, A).
Should the synthesis occur in the tumor, it is extremely interesting to identify the structures responsible for the tumor. As is known, teratocarcinomas are capable of forming differentiated tissues, including the development of a yolk sac and, less frequently, of hepatocytes (Klein-schmit, and Pierce, 1964; Berry et al., 1969). It seems logical, therefore, that it is precisely these tumors, together with hepatomas, that are associated with the occurrence of AFP. We consider it most likely that the AFP synthesis with teratocarcinomas is determined by the formation of a yolk sac, i.e., a structure which is most typical of malignant teratocarcinomas and is able to produce AFP. The problem could be solved unequivocally using the immunofluorescence technique.
Thus, the phenomenon of association of AFP with teratocarcinomas obviously requires experimental studies with "models" and with clinical material, both for its adequate description and for evaluating the prospects of its clinical use.
It was the central objective of this article to emphasize the importance of basic research on the alpha-fetoprotein phenomenon; such studies, aimed to decipher its nature, would contribute to understanding of both normal ontogenesis and pathogenesis of tumors. An essential task in this respect is to elucidate factors which control AFP production during the embryonic period of ontogenesis and resumption of its synthesis in tumors.
Analyzing specific aspects of the phenomenon, we made an attempt to formulate experimental approaches to some immediate problems:
1. Is there any external factor which could control AFP synthesis and determine its intensity?
2. Is the synthesis carried out by certain type of cells, specialized in AFP production, or is this function inherent in any hepatocyte?
3. Is the control of AFP synthesis accomplished by regulation of intensity of the process taking place in individual cells, or by involvement of varying numbers of cells with a constant level of AFP production?
4. Is the AFP synthesis in a tumor due to maintained ability of the stem tumor cell to differentiate, or is it the result of dedifferentiation of the mature hepatocyte?
Answers to these questions are essential, in our opinion, in order to bring the research up to a molecular level.
There is no doubt that elucidation of the AFP phenomenon is facilitated by progress in related fields, as it is not only with tumors of the liver and teratoblastomas that the resumption of the synthesis of embryo-specific proteins takes place. Well known are investigations on the carcino-embryonic antigens of the human digestive system (Gold and Freedman, 1965; Gold et al, 1968; Thomson et al, 1969; Kleist and Burtin, 1970), a phenomenon, which is principally similar to that discussed in the present article. It is not impossible that resumption of the synthesis of embryonic antigens in tumors is a general regularity (Brawn, 1970; Duff and Rapp, 1970; Coggin et al, 1970).
In the practical aspect, the development of a simple and accessible test for AFP with high sensitivity and its trial in a broad epidemiological survey seem to be most important. This approach would help to determine the feasibility of early diagnosis of the liver cancer, at stages permitting surgical treatment.
Since the manuscript was written, several important publications have appeared. A new purification procedure for human AFP has been suggested by Purves et al. (1970a), which includes physiochemical and immunochemical treatments. The AFP preparations obtained were found homogeneous in neutral and alkaline pH ranges, but revealed distinct heterogeneity at pH 4.5. Nishi (1970) has described in detail a method of isolation of human AFP from its precepitate with specific antibody. The purified AFP had S20 = 4.50 S and a molecular weight of 64,000. This is in good agreement with data presented in Table I.
Clinical observations for Japan have been reported in the same paper. AFP was found in 23 patients with hepatocellular carcinoma out of 31 examined; from the histologically confirmed 20 cases there were 16 AFP – positive (80%). No positives were found among 500 patients with noncancerous liver diseases, but one case with AFP was observed among 100 patients with nonliver tumors. The "AFP+" serum belonged to a patient with gastric cancer complicated by massive liver metastases.
One more case of AFP appearance with gastric cancer metastatic to the liver has been reported by Geffroy et al. (1970b).
Purves et al. (1970b) have found that the blood levels of AFP in liver cancer patients reflect to some degree the effect of chemotherapy on the tumor size.
Gitlin and Pericelli (1970) have demonstrated, using the immuno-autoradiography technique, the synthesis of AFP, transferrin, and albumin by human yolk sac.
A direct proof of AFP production by human hepatocellular carcinomas has been obtained by Rioche et al. (1970), who demonstrated AFP accumulation in a medium of cultured liver tumor cells.
References
Abelev, G. I. (1963). Acta Unio Int. Contra. Cancrum 19, 80-82.
Abelev, G. I. (1965a). Progr. Exp. Tumor Res. 7, 104-157.
Abelev, G. I. (1965b). In "Viruses, Cancer, Immunity" (N. Blokhin, ed.), pp. 327-351. Medicine, Moscow.
Abelev, G. I. (1968). Cancer Res. 28, 1344-1350.
Abelev, G. I. (1971). Protides Biol. Fluids, Proc. Colloq. 18 (in press).
Abelev, G. I., and Bakirov, R. D. (1967). Vopr. Med. Khim. 13, No. 4, 378-383.
Abelev, G. I., and Tsvetkov, V. S. (1960). Vopr. Onkol. 6, No. 6, 62-72.
Abelev, G. I., Perova, S. D., Khramkova, N. I., Postnikova, Z. A., and Irlin, I. S. (1963a). Transplantation 1, 174-180.
Abelev, G. I., Perova, S. D., Khramkova, N. I., Postnikova, Z. A., and Irlin, I. S. (1963b). Biokhimiya (Russian) 28, 625-634.
Abelev, G. I., Assecritova, V., Kraevsky, N. A., Perova, S. D., and Perevodchikova, N. I. (1967a). Int. J. Cancer 2, 551-558.
Abelev, G. I., Perova, S. D, Bakirov, R. D., and Irlin, I. S. (1967b). In "Specific Tumor Antigens" (R. J. G. Harris, ed.), UICC Monograph Series, Vol. 2, pp. 32-33. Munksgaard, Copenhagen.
Abelev, G. I., Tsvetkov, V. S, Biryulina, T. I., Elgort, D. A., Olovnikov, A. M., Gusev, A. I., Jazova, A. K., Perova, S. D., Rubzov, I. V., Kantorovich, B. A., Tur, V., Khasanov, A. I., and Levina, D. M. (1971). Byull. Eksp. Biol. Med. No. 4, 75-81.
Alpert, M. (1969). Clin. Res. 17, 461.
Alpert, M., Uriel, J., and de Nechaud, B. (1968). N. Engl. J. Med. 278, 984-986.
Andreoli, M., and Robbins, J. (1962), J. Clin. Invest. 41, 1070-1077.
Annual Report of International Agency for Research on Cancer. (1969). pp. 58-59. IARC, Lyon.
Assecritova, I. V., Abelev, G. I., Kraevsky, N. A., Perova, S. D., and Perevodchikova, N. I. (1967). Vestn. Akad. Med. Nauk SSSR 22, No. 5, 75-81.
Babinov, B. A., and Shain, A. A. (1971). Vopr. Onkol. (in press).
Bagshawe, A., and Parker, A. (1970). Lancet 2, 268.
Bakirov, R. D. (1968). Byull. Eksp. Biol. Med. No. 2, 45-47.
Bakirov, R. D., and Abelev, G. I. (1968). Unpublished experiments.
Baldwin, R. W., and Barker, C. R. (1967). Brit. J. Cancer 21, 338-345.
Basteris, B., Engelhardt, N. V., Gusev, A. I., Prussevich, T., and Luria, E. A. (1971). In preparation.
Beaton, G. H., Selby, A. E., Veen, M. J., and Wright, A. M. (1961). J. Biol. Chem. 236, 2005.
Bergstrand, C., and Czar, B. (1956). Scand. J. Clin. Lab. Invest. 8, 174.
Bergstrand, C., and Czar, B. (1957). Scand. J. Clin. Lab. Invest. 9, 277-283.
Berry, C. L., Keeling, J., and Hilton, C. (1969). J. Pathol. 98, 241-252.
Boffa, G. A., Nadal, C, Zajdela, F., and Fine, J. M. (1964). Nature (London) 203, 1182-1184.
Boffa, G. A., Jagout-Armand, J., Candin-Harding, F., Susbielle, H., and Fine, J. (1965). C. R. Acad. Sci. 159, 1307-1312.
Bourreille, J., Metayer, P., Sanger, F., Matray, F., and Fondimare, A. (1970). Presse Med. 78, 1277.
Brawn, R. I. (1970). Int. J. Cancer 6, 245-249.
Cameron, G. R. (1935). Pathol. Bacteriol. 41, 283-288.
Clark, J. V. (1970). Lancet 1, 88.
Coggin, J., Ambrose, K., and Anderson, N. (1970). J. Immunol. 105, 524-526.
Day, E. (1965). "Immunochemistry of Cancer." Thomas, Springfield, Illinois
de Nechaud, B., and Uriel, K. (1971). Protides. Biol. Fluids, Proc. Colloq. 18 (in press).
de Nechaud, B., Economopoulos, P., and Uriel, J. (1969). Presse. Med. 77, 1945-1947.
Deutsch, H. F. (1954). J. Biol. Chem. 208, 669-678.
Dorfman, N. A. (1967). Vopr. Med. Khim. 13, 267-272.
Duff, A., and Rapp, F. (1970). J. Immunol. 103, 521-523.
Dufour, D., Barrett, D., and Tremblay, A. (1969). Rev. Immunol. Ther. Antimicrob. 33, 315-321.
Economopoulos, P., Theodoropoulus, G., and Sakellaropoulos, N. (1970). Lancet 1, 1337.
Elgort, D. A., and Abelev, G. I. (1971). Byull. Eksp. Biol. Med. No. 2, 118-120.
Endo, Y. et al. (1969). Naika Hokan 14, 24-28.
Engelhardt, N. V. (1970). Unpublished experiments.
Engelhardt, N. V., Shipova, L. Ja., Gusev, A. I., Jazova, A. K., and Ter-Grigorova, E. N. (1969). Byull. Eksp. Biol. Med. No. 12, 62-64.
Engelhardt, N. V., Gusev, A. I., Shipova, L. Ja., and Abelev, G. I. (1971). Int. J. Cancer 7, 198-206.
Fahan, F., Kropackova, L., Sirlova, A., and Magrot, T. (1966). Naturwissenschaften 53, 555-556.
Foli, A., Sherlock, S., and Adinolfi, M. (1969). Lancet 2, 1267-1269.
Foy, H., Gillman, T., Kondi, A., and Preston, J. (1966). Nature (London) 212, 150.
Foy, H., Kondi, A., Linsell, C, Parker, A., and Sizaret, P. (1970a). Nature (London) 225, 952-953.
Foy, H., Kondi, A., and Linsell, C. (1970b). Lancet 1, 411.
Foy, H., Kondi, A., Parker, A., Stenley, R., and Venning, C. (1970c). Lancet 1, 1336.
Foy, H, Kondi, A., and Linsell, C. (1970d). Lancet 2, 663.
Galdo, A., Casado, J., and Talavera, R. (1959). Arch. Fr. Pediat. 16, 954-962.
Geffroy, Y., Denis, P., Colin, R., Sanger, F., Matray, F., and Fondimare, A. (1970a). Presse Med. 78, 1107-1108.
Geffroy, Y., Metayer, P., Denis, P., Phillip, J., Matray, F., Sanger, F., Laumonier, R., and Duval, C. (1970b). Presse Med. 78, 1896.
Gitlin, D., and Boesman, M. (1966). J. Clin. Invest. 45, 1826-1838.
Gitlin, D., and Boesman, M. (1967a). Comp. Biochem. Physiol. 21, 327-336.
Gitlin, D., and Boesman, M. (1967b). J. Clin. Invest. 46, 1010-1016.
Gitlin, D., and Pericelli, A. (1970). Nature 228, 996-997.
Gitlin, D., Kitzes, J., and Boesman, M. (1967). Nature (London) 215, 534.
Gold, P., and Freedman, S. (1965). J. Exp. Med. 122, 464-481.
Gold, P., Gold, M., and Freedman, S. (1968). Cancer Res. 28, 1331-1334.
Grabar, P., Stanislawski-Birencwajg, M., Oisgold, S., and Uriel, J. (1967). In "Specific Tumor Antigens" (R. J. C. Harris, ed.), UICC Monograph Series, Vol. 2, pp. 20-32. Munksgaard, Copenhagen.
Guelstein, V. I. (1966). Dissertation Thesis, Moscow.
Guelstein, V. I., and Khramkova, N. I. (1965). Neoplasma 12, 251-260.
Gusev, A. I., and Jazova, A. K. (1970a). Biokhimiya 35, 172-181.
Gusev, A. I., and Jazova, A. K. (1970b). Byull. Eksp. Biol. Med. No. 4, 120-122.
Gusev, A. I., Jazova, A. K., and Poljakova, E. (1971a). Byull. Eksp. Biol. Med. No. 3, 69-72.
Gusev, A. I., Engelhardt, N. V., Masseyeff, R., Camain, R., and Basteris, B. (1971b). Int. J. Cancer 7, 207-217.
Hamashima, J., Harter, J., and Coons, A. (1964). J. Cell Biol. 20, 271-279.
Herrich, H., and Lawrence, J. (1965). Anal. Biochem. 12, 400-402.
Hochwald, G., Thorbecke, G., and Asofsky, R. (1961). j. Exp. Med. 114, 459.
Houstek, J., Masopust, J., Kithier, K., and Radl, J. (1968). J. Pediat. 72, 186-193.
Hull, E., Moertel, C, and Carbone, P. (1969a). Clin. Res. 17, 403.
Hull, E,, Carbone, P, Gitlin, D., O'Gara, R., and Kelly, M. (1969b). J. Nat. Cancer Inst. 42, 1035-1044.
Hull, E., Carbone, P., O'Gara, R., Moertel, C, O'Conor, G., and Smith, C. (1969c). I ARC Conf. Primary Liver Cancer, London (unpublished).
Hull, E., Carbone, P, Moertel, C, and O'Conor, G. (1970). Lancet 1, 779.
Irlin, I. S., Perova, S. D., and Abelev, G. I. (1966). Int. J. Cancer 1, 337-347.
Jazova, A. K., and Gusev, A. I. (1971). Byull. Eksp. Biol. Med. (in press).
Kahan, B., and Ephrussi, B. (1970). J. Nat. Cancer Inst. 44, 1015-1036.
Khasanov, A. I., Abelev, G. I., Perova, S. D., Polenko, V. K., Ryaposova, U. K., and Shirenkova, O. (1971). Vopr. Onkol. (in press).
Khramkova, N. I., and Abelev, G. I. (1961). Byull. Eksp. Biol. Med. No. 12, 107-111.
Khramkova, N. I., and Guelstein, V. I. (1965). Neoplasma 12, 239-250.
Khramkova, N. I., and Guelstein, V. I. (1967). Unpublished data.
Kirsh. I., Wise, R., and Oliver, I. (1967). Biochem. J. 102, 763-766.
Kithier, K., and Prokes, J. (1966). Biochim. Biophys. Acta 127, 390-399.
Kithier, K., Houstek, J., Masopust, J., and Radl, J. (1966). Nature (London) 212, 414.
Kithier, K., Masopust, J., and Radl, J. (1968a). Biochim. Biophys. Acta 160, 135-137.
Kithier, K., Valenta, Z., and Zizkovsky, V. (1968b). Conference on Veterinary Pathology, Brno (unpublished).
Kleinschmit, L., and Pierce, B. (1964). Cancer Res. 24, 1544-1552.
Kleist, S., and Burtin, P. (1970). In "Immunity and Tolerance in Oncogenesis" (L. Severi, ed.), pp. 286-296. Perugia.
Kohn, J., and Muller, U. (1970). Lancet 1, 142. Kresno, S., Gandasoebrata, K., and Rumke, P. (1970). Lancet 1, 1178.
Lawford, P. J. (1961). Biochem. Pharmacol. 7, 109.
Luria, E. A., Bakirov, R. D., Yeliseyeva, T. A., Abelev, G. I., and Fridenstein, A. I. (1969). Exp. Cell Res. 54, 111-117.
McKellar, M. (1949). Amer. J. Anat. 85, 263.
Masopust, J. (1966). "Ontogeny of Human Serum Proteins," Vol. 45. Babakova Sbirka, Prague.
Masopust, J., Kithier, K., Fuchs, V., Kotal, L., and Radl, J. (1967). In "Intrauterine Dangers to the Foetus" (J. Horsky and Z. Stembera, eds.), pp. 30-35. Excerpta Med. Found., Amsterdam.
Masopust, J., Kithier, K., Radl, J., Koutecky, J., and Kotal, L. (1968). Int.. J. Cancer 3, 364-373.
Masopust, J., Zizkovsky, V., and Kithier, K. (1971). Protides Biol. Fluids, Proc. Colloq. 18 (in press).
Masseyeff, R. (1971). Protides Biol. Fluids, Proc. Colloq. 18 (in press).
Masseyeff, R., Sankale, M., Onde, M., Menye, A., Camain, R., Quenum, C, Magdat, L., Mattern, P., Ancelle, I., and Leblanc, L. (1968). Bull. Soc. Med. Ajr. Noire Langue Fr. 13, 537-548.
Massiukevitch, V. (1970). Dissertation Thesis, Astrakhan (USSR). Mawas, C, Buffe, D., Lemerle, J., Schweisguth, O., and Burtin, P. (1969a). Arch. Fr. Pediat. 26, 779.
Mawas, C, Kohen, M., Lemerle, J., Buffe, D., Schweisguth, 0., and Burtin, P. (1969b). Int. J. Cancer 4, 76-79.
Mawas, C, Buffe, D., and Burtin, P. (1970). Lancet 1, 1291.
Mawas, C, Buffe, D., Schweisguth, O., and Burtin, P. (1971). Protides Biol. Fluids., Proc. Colloq. 18 (in press).
Monjour, L., and Mariage, C. (1969). C. R. Soc. Biol. 163, 1288-1290.
Morkhov, J. K., and Sokolov, J. N. (1970). Khirurgia (Moscow) No. 2, 133-136.
Morris, H., Dyer, H., Wagner, B., Myah, H., and Rechigl, M. (1964). Advan. Enzyme Regul. 2, 321-333.
Muralt, P., and Roulet, D. (1961). Helv. Paediat. Acta. 16, 517-533.
Nishi, S. (1970). Cancer Res. 30, 2507-2513.
Nishi, S., Watanabe, H., Tsukada, J., and Hirai, H. (1971). Protides Biol. Fluids, Proc. Colloq. 18 (in press).
Nordmann, Y., and Shapira, F. (1967). Eur. J. Cancer 3, 247-250.
O'Conor, G. T., Tatarinov, Ju. S., Abelev, G. I., and Uriel, J. (1970). Cancer 25, 1091-1098.
Olovnikov, A. M. (1967). Immunochemistry 4, 77-80.
Olovnikov, A. M., and Tsvetkov, V. S. (1969). Byull. Eksp. Biol. Med. No. 12, 102-104.
Pantelouris, E., and Hale, P. (1962). Nature (London) 195, 79.
Pedersen, K. (1944). Nature (London) 154, 575.
Perova, S. D. (1969). Dissertation Thesis, Moscow.
Perova, S. D., and Abelev, G. I. (1967). Vopr. Med. Khim. 13, 369-377.
Perova, S, D., Elgort, D. A., and Abelev, G. I. (1971). Byull. Eksp. Biol. Med. No. 3, 45-47.
Portugal, M. L., Azevedo, M. S., and Manso, C. (1970). Int. J. Cancer 6, 383-387.
Purves, L., Machab, M., and Bersohn, I. (1968). S. Ajr. J. Med. 42, 1138-1141.
Purves, L., Machab, M., Rolle, M., and Bersohn, I. (1969). S. Ajr. J. Med. 44, 1194-1196.
Purves L. R., Van der Merwe, E., and Bersohn, I. (1970a). South Ajr. J. Med. 44, 1264-1268.
Purves, L. R., Bersohn, I., Geddes, E., Falkson, G., and Cohen, L. (1970b). South Ajr. J. Med. 44, 590-594.
Purves, L. R., Bersohn, I., and Geddes, E. W. (1970c). Cancer 25, 1261-1270.
Reuber, M., and Glover, E. (1970). J. Nat. Cancer Inst. 44, 419-127.
Rioche, M., Quelin, S., Seek, J., Jacquesson, M., Basteris, B., and Masseyeff, R. (1970). C. R. Acad. Sci. (Paris) 271, 1148-1151.
Rosentahl, M., Wishnow, R., and Sato, G. (1970). J. Nat. Cancer Inst. 44, 1001.
Rowe, D. S. (1969). Bull. W. H. O. 40, 613.
Rowe, D. S. (1970a). Lancet 1, 1340.
Rowe, D. S. (1970b). WHO Immunoglobulins Reference Center, Lausanne. Personal communication.
Sainte-Marie, G. (1962). J. Histochem. Cytochem. 10, 250-256.
Schapira, F., Dreyefus, J., and Schapira, G. (1963). Nature (London) 200, 995-997.
Schapira, F., Reuber, M., and Hatzfeld, A. (1970). Biochem. Biophys. Res. Commun. 40, 321-327.
Shipova, L. Ja., Abelev, G. I., Gusev, A. I., and Engelhardt, N. V. (1971). In preparation. Smith, J. B., and Blumberg, B. S. (1969). Lancet 2, 953.
Smith, J. B., and Todd, D. (1968). Lancet 2,833.
Spiro, R. (1960). J. Biol. Chem. 235, 2860-2869.
Stanislawski-Birencwajg, M. (1965). C. R. Acad. Sci. 260, 364-366.
Stanislawski-Birencwajg, M. (1967). Cancer Res. 27, 1982-1989.
Stanislawski-Birencwajg, M., Uriel, J., and Grabar, P. (1967). Cancer Res. 27, 1900-1907.
Tatarinov, Ju. S. (1964a). Vopr. Med. Khim. 10, 90-91.
Tatarinov, Ju. S. (1964b). Vopr. Med. Khim. 10, 218-219.
Tatarinov, Ju. S. (1964c). Vopr. Med. Khim. 10, 584-588.
Tatarinov, Ju. S. (1965a). Vopr. Med. Khim. 11, No. 2, 20-24.
Tatarinov, Ju. S. (1965b). Vopr. Med. Khim. 11, No. 3, 98-99.
Tatarinov, Ju. S. (1967). Vopr. Med. Khim. 13, 37-42.
Tatarinov, Ju. S. (1968). Nature (London) 217, 964-965.
Tatarinov, Ju. S. (1970). Vestn. Akad. Med. Nauk SSSR 25, No. 9, 68-72.
Tatarinov, Ju. S,, and Afanassieva, A. V. (1965). Byull. Eksp. Biol. Med. No. 6, 65-59.
Tatarinov, Ju. S., and Nogaller, A. I. (1966). Vopr. Onkol. 12, No. 12, 26-30.
Tatarinov, Ju. S., Massiukevitch, V. N., Mesnyankina, N. V., and Parfenova, L. F. (1967). Akusherstvo i Ginecol. (USSR) No. 8, 20-22.
Thomson, D., Krupey, J., Freedman, S., and Gold, P. (1969). Proc. Nat. Acad. Sci. 64, 161-167.
Uriel, J. (1969). Pathol. Biol. 17, 877-884.
Uriel, J., de Nechaud, B., Stanislawski-Birencwajg, M., Masseyeff, R., Leblanc, L., Quenum, C, Loisillier, F. and Grabar, P. (1967). C. R. Acad. Sci. 265, 75-78.
Van Furth, R., and Adinolfi, M. (1969). Nature (London) 222, 1296-1299.
Van Gool, J., and Ladiges, N. (1969). j. Pathol. 97, 115-126.
Vasileysky, S. S. (1966). Biokhimiya 31, 959-961.
Vasileysky, S. S., and Yablokova, V. (1964). Byull. Eksp. Biol. Med. No. 4, 52-54.
Weimer, H., and Benjamin, D. (1965). Amer. J. Physiol. 209, 736-745.
Wise, R., and Oliver, J. (1966). Biochem. J. 100, 330-333.
Wise, R., Ballard, F., and Ezekiel, E. (1963). Comp. Biochem. Physiol. 9, 23.
Zizkovsky, V., Masopust, J., and Prokes, J. (1971). Protides Biol. Fluids, Proc. Colloq. 18 (in press).