| Home page (in Russian) | Texts in English | Guestbook |
Transplantation 1:174–180, 1963
G. I. Abelev, S. D. Perova, N. I. Khramkova, Z. A. Postnikova and I. S. Irlin
Department of Immunology and Oncology
Gamaleya Institute of Epidemiology and Microbiology
Moscow, U.S.S.R.
|
SUMMARY It was previously reported that one strain of mouse hepatoma (strain XXIIa) contained an antigenic substance which was absent from liver, spleen, kidney, lung or serum of normal adult mice (1, 13). This antigen was isolated in an immunologically pure state (2, 3). It reacted only with anti-hepatoma sera and did not precipitate with antisera against liver, spleen, kidney or against normal adult mouse sera. The present work, however, demonstrates that this antigen is a normal constituent of the embryo and of newborn mouse serum, and that it disappears from the serum as the mice become older. It also shows that the same antigen reappears in the serum of adult hepatoma-bearing mice as a result of its synthesis in the tumor tissue followed by its secretion into the blood. Judged by its electrophoretic mobility this antigen is related to the α-globulin fraction of mouse serum. |
Our experiments were carried out mainly with hepatoma strain XXIIa; this tumor was induced in 1951 in C3H-A mice by the action of orthoaminoazotoluene, and it has been maintained within the same line of mice (7). Three other hepatoma strains were originally induced by the same carcinogen in the same line of animals: strain XXII is the substrain of XXIIa, strain XXXVIII was induced in 1960, and hepatoma XLVI in 1961 (8).
Two antisera against newborn mouse sera (anti-NB) were obtained by immunization of rabbits as follows: 3 injections, each consisting of 3 ml, were given at intervals of two and one weeks, respectively; the first of these injections was with complete Freund's adjuvant and the other two without it. Blood was taken one week after the last injection. Two sera against adult mouse serum (anti-AS) were prepared in the same way. The preparation of antihepatoma sera has been described previously (1, 13). All analyses were performed by the agar-precipitation microtechnique (10) and by the immunoelectrophoresis method (9) in semi-micro-modification (2, 12).
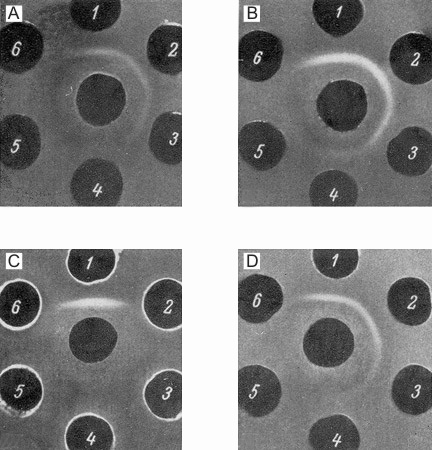
The presence of the specific antigenic component in the sera of newborn mice and its estimation in different systems was carried out in the following way. The anti-NB-serum was absorbed by an equal volume of adult mouse serum (AS) and after discarding the precipitate formed, it was tested with different dilutions of newborn mouse sera (NB). The precipitin line which occurred in this system was formed by the NB-dilutions up to 1/2000. The optimal reaction was seen between absorbed anti-NB and NB diluted 1/256. These components formed the standard test system used for further investigations. In each experiment the above test system was compared with a series of two-fold dilutions of the system to be tested (Fig. la-d). It was possible in this way to reveal the presence of a certain antigen in the systems compared, as well as to determine the end-point of titration of this component in different systems. Figure 2 presents the summarized data concerning the determination of this antigen in the sera of mouse embryos (second part of pregnancy) and of newborn and postnatal mice up to adult life, and also in the sera of adult mice grafted with hepatoma XXIIa (14-21 days after transplantation). All data were obtained with the same standard test system. The levels of the antigen studied were given in arbitrary units.

| Figure 1. Estimation of embryonal serum antigen in the sera of mice in different periods after birth and in the serum of a hepatoma-bearing mouse. In central wells of all figures anti-NB-sera absorbed by the sera of adult mice. Well 1: newborn mouse sera diluted 1/256; Wells 2-6: two-fold dilutions of the samples tested. 1A: sera of newborn mice (from 1/512 to 1/8192); 1B: serum, of one-week old mouse (1/128-1/2048); 1C: serum of adult mice (l-l/16) / (antigen is not detectable); 1D: serum of hepatoma-bearing mouse (1/128-1/2048). |
The inverse value of the end-point of titration of the embryo serum was taken as 100%. From 2 to 14 individual sera were used for each determination and average values are given in Figure 2. The data on embryo and NB sera were obtained on the serum pool from 9 and 40 animals, respectively. Embryo serum gave a precipitin line up to a dilution of 1/1000. The negative results obtained with the sera of adult mice therefore mean that the component studied is absent, or present in concentrations lower than 0.025% of its level in embryo sera.
From the data presented in Figure 2 it is obvious that the concentration of the antigen studied gradually decreases during the first three weeks after birth and that, subsequently, this antigen disappears from the sera. The antigen is present in all mouse lines tested and its presence depends only on the age of the animals. A significant accumulation of the antigen was obtained in the sera of tumor-bearing mice after the transplantation of hepatoma XXIIa.
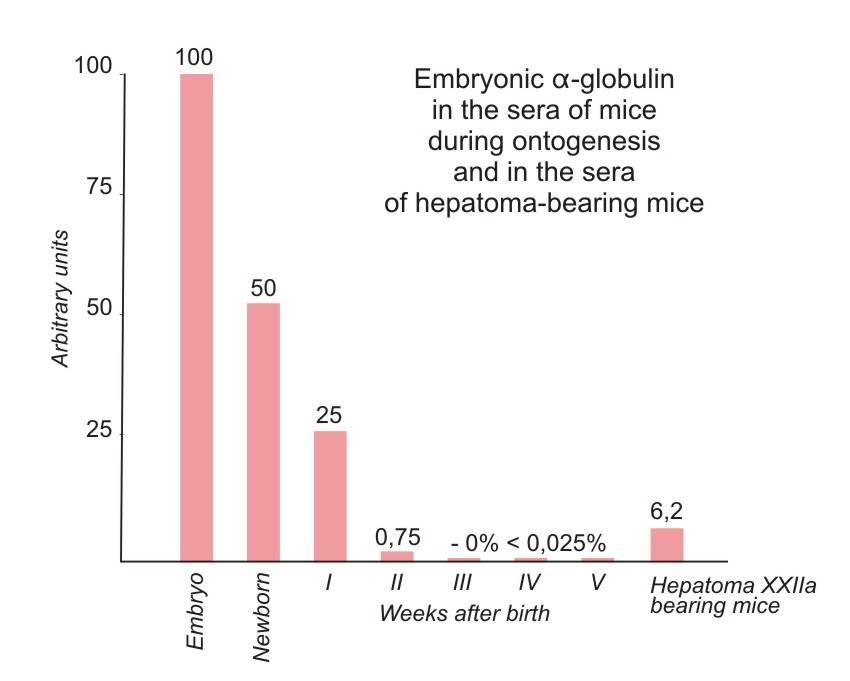
| Figure 2. Relative concentrations of the antigen in the sera of mice in different periods of development and of mice grafted with hepatoma XXIIa. All the titrations were made with the anti-NB-serum absorbed by the adult mouse serum (see Fig. 1). The concentration of the antigen giving the end-point 1/4096 was taken as 100%. Negative reaction with the test system used means that the antigen is absent or present in concentration lower than 0.025%. |
To carry out a more thorough comparison of the antigens detected in the embryo sera and in the sera of hepatoma-bearing mice the isolation of the antigen from both sources as well as from hepatoma extract was performed. Two ml of NB were subjected to electrophoresis in agar-gel (agar-block dimensions, 15 X 11 X 1 cm; barbituric acid-sodium barbiturate buffer at pH 8.6; ionic strength (μ) = 0.025) at the gradient potential 4v/cm during 6 hours) in the apparatus described previously (12). The fraction containing the antigen studied after electrophoresis was located in the α-globulin region and after elution from the agar sections (12) contained the characteristic antigen together with some other antigens common for NB and AS. For the elimination of these admixtures the immunofiltration method (2,3) was used: the α-globulin fraction of NB or of the sera of hepatoma-bearing mice was again subjected to electrophoresis in agar, but in this case the γ-globulin fraction from the anti-AS was placed in the pathway of antigen migration. During electrophoresis the antigens with anodic mobility met the γ-globulins which moved by electro-osmosis in the opposite direction. The components reacting with anti-AS antibodies were precipitated while the specific antigen of NB serum passed the antibody "filter" without changing its mobility and could therefore be eluted from the agar in immunologically pure form. The detailed description of this technique has been given elsewhere (2, 12). When isolating the antigen from the extract of hepatoma XXIIa the mixture of anti-AS and anti-liver sera was used as a specific "filter".
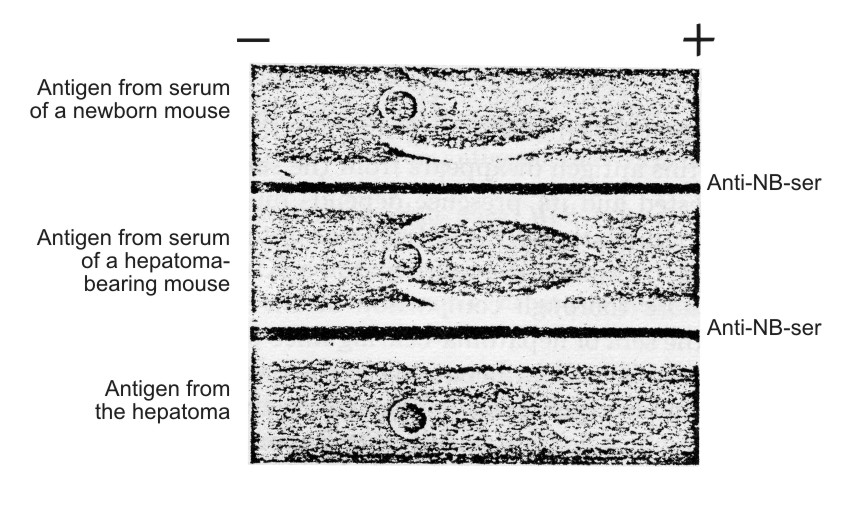
Antigenic preparations isolated from three different sources were identical as judged by agar-precipitation and immunoelectrophoresis techniques (Figs. 3 and 4) using unabsorbed anti-NB. It was possible, using the immunoelectrophoresis technique developed by Osserman (11), to identify the purified antigen with a certain α-globulin line on the gel-diffusion plate after the immunoelectrophoresis of the NB (Fig. 5).
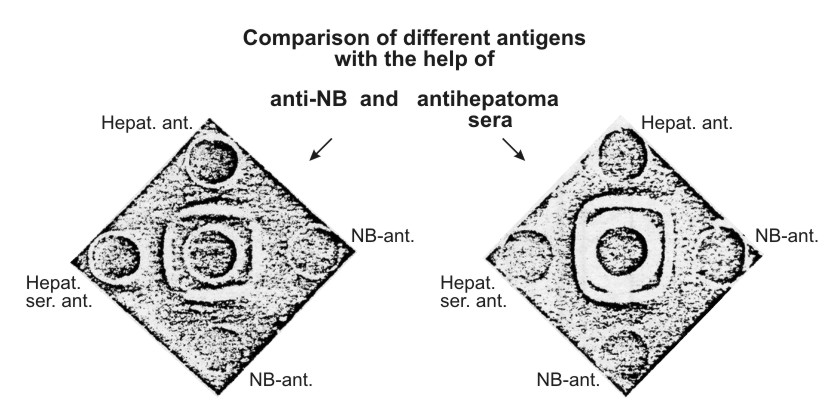
| Figure 3. Comparison of the characteristic antigen isolated from newborn mouse sera (NB-ant.), sera of mice with hepatoma XXIIa (Hepat. ser. ant.), and from the extract of this hepatoma (Hepat. ant.) Left figure: central well – unabsorbed anti-NB-serum. Right figure: central well – unabsorbed anti-hepatoma XXIIa serum. Reaction of identity is clearly seen between all the preparations tested. |
| Figure 4. Immunoelectrophoresis of antigens isolated from hepatoma XXIIa extract, from sera of hepatoma-hearing mice and from newborn mouse sera (95 min electrophoresis in 1% agar on barbituric acid-sodium barbiturate buffer, pH 8.6; μ = 0.025 at 5-5.5 v/cm). Unabsorbed anti-NB-serum used. |
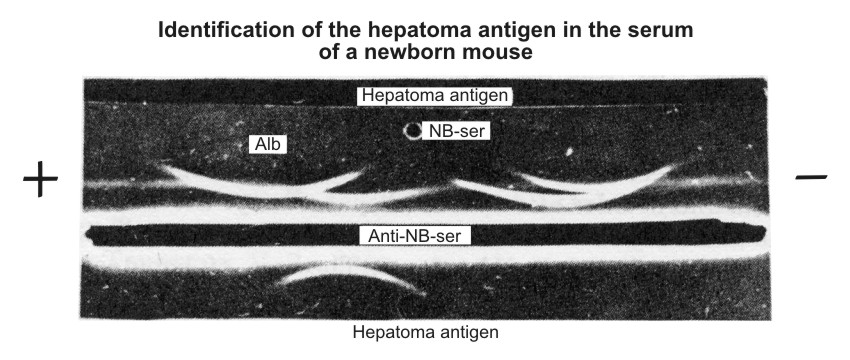
| Figure 5. Identification of characteristic hepatoma antigen in the serum of newborn mice according to Osserman technique (11). NB-ser. – serum of newborn mice diluted I/4. Anti-NB-ser. – rabbit unabsorbed antiserum against the sera of newborn mice. Hepatoma antigen – the antigen isolated from the sera of hepatema-bearing mice. The straight line formed by the "Hepatoma antigen," placed in the upper trench, and anti-NB-serum (lower trench) fused with the are of antigen displaced in the α-globulin region of NB-serum. |
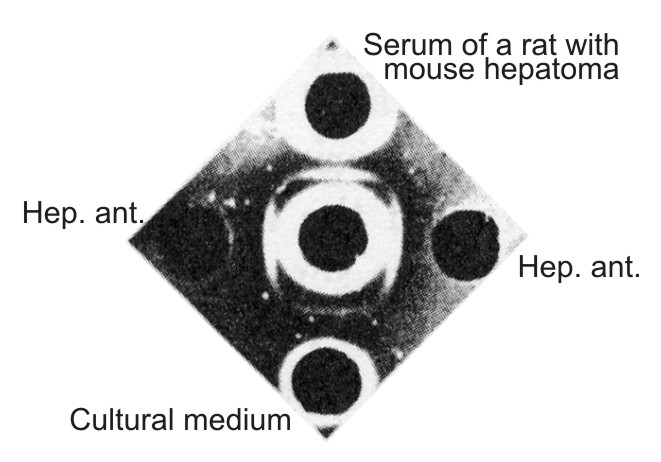
| Figure 6. Comparison of the mouse hepatoma antigen with the antigen from the serum of a rat grafted with mouse hepatoma and with the antigen from the tissue culture medium. In the center well – antiserum against serum of newborn mice absorbed by serum of adult mice. Hep. ant. – hepatoma XXIIa extract taken in an optimal dilution to the absorbed antiserum. In upper well – undiluted serum of cortisone-treated rat grafted with hepatoma XXIIa 15 days previously. In lower well – a liquid phase of primary hepatoma XXIIa culture taken 16 days after the explantation in vitro. All preparations give the identity reaction. |
The next and most important problem was connected with the determination of the site of origin of the antigen in the hepatoma-bearing mice. Since the level of the antigen in the blood of the hepatoma-bearing animals was several times higher than in tumor extract the question arises: is it formed in the tumor itself or does it merely accumulate in the tumor after being formed in some other tissue as a responce to the tumor graft? The latter possibility seemed plausible because there are many studies demonstrating some changes in the α-globulins connected with tumor growth (4, 5, 6). To detect the site of antigen production, experiments with heterotransplantation of mouse hepatoma into cortisone-treated rats and cultivation of tumor tissue in vitro were performed. In both cases formation and secretion of mouse hepatoma antigen were demonstrated.
The antigen found in the sera of rats grafted with mouse hepatoma and in tissue culture medium were serologically identical with the characteristic antigen present in mouse hepatoma extract (Fig. 6) or in NB sera. In primary tissue cultures, continuing accumulation of the antigen in the medium which paralleled the growth of monolayer was demonstrated. Moreover, we now have at our disposal the fifth generation of tissue culture originally derived from the ascitic subline of (XXIIa) hepatoma which retained the ability for active production of embryonal α-globulin as well as of mouse serum albumin. Thus it seems that the embryonal α-globulin which appeared in the blood of hepatoma-bearing mice is formed in the tumor and then secreted into the blood.
We also investigated the sera of: a) mice grafted with three other slowly growing hepatoma strains (XXII, XXXVIII and XLVI)*; b) partially hepatectomised mice; and c) mice grafted with 20 different non-hepatic tumors. This last group included such tumors as the Ehrlich carcinoma, the Crocker and 37 sarcomas, several sarcoma strains induced by methylcholanthrene and benz-anthracene, 5 transplantable leukoses, 2 mammary gland tumors, one stomach tumor and two skin carcinomas. One or two sera from individual mice with each kind of tumor were tested. Slight accumulation of the antigen was demonstrated in sera of mice with slowly growing hepatomas (XXII and XXXVIII) and in the serum of mice with regenerating liver 48 hours after the operation (Fig. 7). One hepatoma (XLVI) and all the non-hepatic tumors gave negative results.
|
* 6-9 animals in each group. |
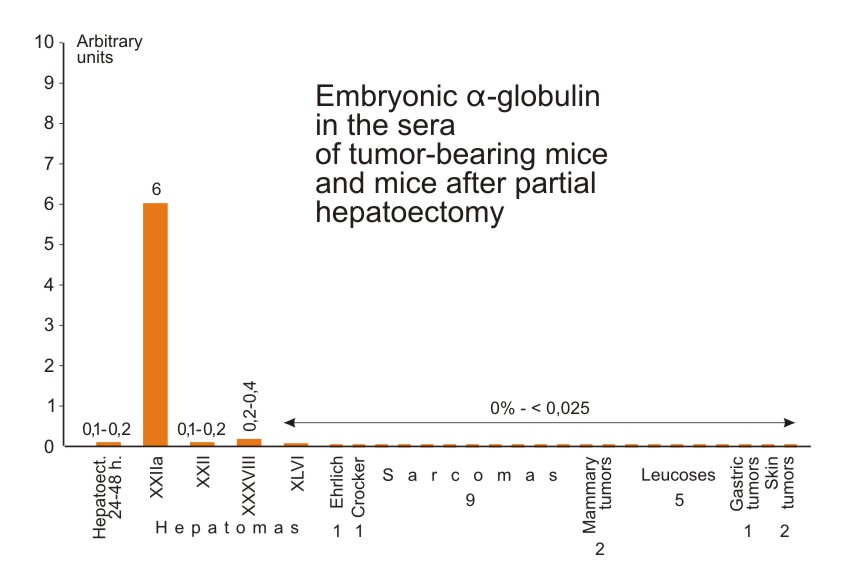
| Figure 7. Relative concentrations of the characteristic antigen in sera of mice grafted with different tumors and of mice after partial hepatectomy. Method of determination of the antigen and the arbitrary units chosen are the same as in Figure 2. The sera were taken from mice with palpable well developed tumors. The sera of hepatectomized mice were tested 24 and 48 hours after the hepatectomy. |
The production of embryonal α-globulin was therefore observed only when proliferation of normal or malignant hepatic cells was taking place. We had the impression that the production of antigen was proportional to the growth rate of hepatoma tissue. Thus, the antigen previously found in mouse hepatoma is not a new substance unique for malignant tissue. The mechanism of its synthesis is present but it is repressed in normal liver cells, and activation of this mechanism takes place in some transplantable hepatic tumors. This activation is probably caused by the transition of tissue from the resting state to proliferation.
By using similar techniques it was possible to demonstrate a similar (but rat-specific) α-globulin in the sera of newborn rats which could not be detected in the sera of adult rats but which accumulated in ascitic fluid and in the sera of adult rats grafted with rapidly growing ascitic rat hepatoma (hepatoma Zajdela). Thus it may suggested that the phenomenon described is common to different transplantable hepatomas of mice and rats. Further work on the study of the substances and the phenomenon described is in progress.
REFERENCES
Received August 26, 1962.

Michael B. Shimkin. Some Classics of Experimental Oncology (1775 – 1965). Pages 601–607

"To render honor to Prof. G. I. Abelev..." (PDF-file)