|
• Alpha-Fetoprotein in Ontogenesis and its Assotiation with Malignant Tumors (1971) |
Int. J. Cancer: 11, 448-459 (1973)
Localization of Alpha-Fetoprotein
In Transplantable Murine Teratocarcinomas
by
N. V. Engelhardt, V. S. Poltoranina and A. K. Yazova
Tumor Immunochemistry and Diagnosis Laboratory, N. F. Gamaleya Institute for
Epidemiology and Microbiology, USSR Academy of Medical Sciences, Moscow, USSR
Received: September 18, 1972, and in revised form December 4, 1972
Embryo-specific serum component α-fetoprotein (AFP) was localized by the indirect immunofluorescent technique on paraffin sections of transplantable teratocarcinoma of strain 129/SV-SI CP mice. AFP was found in several types of structures comprising an insignificant part of the total section area. Most frequent and bright AFP-specific fluorescence occurred in the epithelium of endodermal origin lining cysts and tubules; in addition, AFP was found in sheets of cells, groups or individual cells of non-identified origin and in squamous epithelium arranged in pearls. In cysts and granules isolated from the ascitic fluid, developing after an intraperitoneal injection of teratocarcinoma suspension, AFP was found in cells of the external layer, very similar to those of the visceral yolk sac, and in the embryotype mesenchyme inside the cysts. By analytical disc-electrophoresis in polyacrylamide gel, cysts were found to contain albumin, AFP, transferrin and gamma-globulin, the relative proportion of AFP being considerably higher than in the ascitic fluid as compared with the other proteins mentioned. It is suggested that AFP localization in teratocarcinomas corresponds to the sites of its synthesis in the embryo and that AFP synthesis is a specific function of certain forms of endodermal differentiation.
It is well established that two forms of tumors – hepatocellular cancer and teratocarcinomas of animals and human beings – are regularly accompanied by reappearance in the serum of embryo-specific component – α-fetoprotein (AFP). AFP is the main component of embryonal sera of various mammalian species. It can easily be distinguished from other fetal serum proteins by its physico-chemical and especially immunological properties: AFP of different species share common antigenic determinants (Abelev, 1971). During normal ontogenesis AFP is synthetized by the yolk sac and liver of fetuses (Gitlin and Boesman, 1966; Abelev, 1965; Wise and Oliver, 1966; Abelev and Bakirov, 1967; Abelev, 1971).
In the tumor-bearing animals and in cancer patients the AFP synthesis is effected by tumor cells proper (Abelev et al., 1963; Kahan and Levine, 1971; Abelev, 1971). However, the cellular basis of AFP production in tumors is not clear because of insufficient direct experimental data. What cells are responsible for AFP synthesis in teratocarcinomas?
Teratocarcinomas are very peculiar neoplasms. They are composed of embryonal carcinoma cells and various somatic tissues representing derivatives of all three germinal layers. In the case of murine teratocarcinomas, the stem cell is a multipotential embryonal carcinoma cell which on the one hand provides for tumor growth and on the other hand differentiates into the somatic tissues of teratocarcinomas (Pierce et al., 1959, 1960; Kleinsmith and Pierce, 1964).
Cells analogous to hepatocytes are very rarely found in human teratocarcinomas, and in those of mice they have not been described at all (Stevens and Hummel, 1957). It has been suggested, therefore, that AFP synthesis in teratocarcinomas is carried out by structures analogous to yolk sac, which are rather common in these tumors (Abelev, 1971; Gitlin et al., 1972). Other authors (Kahan and Levine, 1971) considered the non-differentiated cells of embryonal carcinoma as a possible site of AFP synthesis.
The immunofluorescence technique seemed to be a useful approach to estimate the cells connected with the AFP-production. The only previous report about AFP localization in teratocarcinomas is that of Mawas et al. (1969). In the frozen sections of human teratocarcinoma bright AFP-specific fluorescence was observed in the cytoplasm of pseudostratified epithelium lining a cyst, and faint fluorescence was present in the solid zones of the tumor. In the metastasis to a lymph node characterized as embryonal epithelioma, AFP was found in some of the cells distributed in groups or trabecules. No further characterization of AFP-containing structures was reported.
In the present study localization of AFP has been investigated in transplantable murine teratocarcinomas. This experimental model is very similar to human primary teratocarcinomas (Stevens and Hummel, 1957; Stevens, 1958).
Materials and Methods
Mice
A special subline of inbred strain 129, designated 129/SV-SL CP, was used. These mice were kindly provided by Dr. L. C. Stevens of Jackson Laboratory, Bar Harbor, Maine, USA.
Transplantable teratocarcinoma O TT 6050
This neoplasm was also provided by Dr. L. C. Stevens. The initial tumor was derived from a 6-day embryo grafted to the testis of an adult mouse in 1967 and subsequently transplanted subcutaneously in males by tissue suspension in saline at 25- to 35-day intervals.
Five consecutive generations were examined during this study. In all cases the tumor sections besides the embryonal carcinoma contained different tissues. Most prominent was nervous tissue with pathological nervous tubules. Also present in these sections were muscle tissue sometimes resembling the heart muscle, cartilage, bone, numerous glands, tubes and cysts lined with different types of epithelium, resembling that of intestine, or visceral yolk sac, or ectodermal epithelium with keratinization, bronchogenic or squamous epithelium.
Free-floating bodies
Free-floating bodies were obtained in a group of animals according to Pierce et al. (1959). These authors found that mice of the 129th strain, if injected intraperitoneally with a suspension of subcutaneous teratocarcinoma, develop solid tumors, hemorrhagic ascites, and, in the majority of cases, numerous cysts and small granules floating in the ascitic fluid. Cells of the external layer covering the cysts and granules very closely resembled those of the visceral yolk sac. We exploited this route of inoculation in order to increase the number of such cells in the tumor and study their relation to AFP synthesis in teratocarcinomas. In four cases when cysts and granules were present they were collected from the ascites, washed in Petri dishes in several changes of large volumes of Ringer solution, fixed, dehydrated, and embedded in paraffin with wax by the same procedures as tumor specimens.
Medium from the cysts
Larger cysts (diameter 3-5 mm) were placed after washing onto a dry region of a Petri dish and punctured with thin glass capillaries. The internal liquid was collected and diluted in 0.25 ml of Tris-HCl buffer, pH 6.7 (1:20-l:40), centrifuged for 15 min at 13,000 rpm and subjected to analytical polyacrylamide gel disc-electrophoresis.
Antibodies against AFP
Antibodies were prepared and checked for monospecificity as described previously (Engelhardt et al., 1971). They reacted only with AFP in agar precipitation and were completely neutralized in immunofluorescence after addition of a slight excess of purified murine AFP.
Antibodies against γG
These antibodies were as described in a previous publication (Engelhardt et al., 1971).
Immunomorphological localization
This was performed on serial paraffin sections of tissues fixed with ethanol-acetic acid. Treatment of tissue specimens, as well as the technique of immunofluorescence, was described in detail earlier (Engelhardt et al., 1971).
Histological examination
For histological examination, we used either the sections employed for immunofluorescence localization AFP after disembedding and staining with hematoxylin-eosin, or consecutive serial sections.
Analytical polyacrilamide gel disc-electrophoresis
This was performed according to Davis (1964) in 4% and 7% gels with slight modifications (Goussev, 1969; Goussev and Yazova, 1970). The diluted media from large cysts and corresponding ascitic fluid diluted 1:40 were separated in the same run for comparison.
Electrophoretic fractions eluted after separation from different regions of the column were identified by agar precipitation using standard test-systems for mouse AFP, albumin, transferrin and γG (Goussev and Yazova, 1970).
AFP concentration
AFP concentration in sera and ascitic fluids of mice bearing teratocarcinomas was determined using the standard agar precipitation procedure with the test-system (Abelev and Bakirov 1967).
Results
AFP in solid teratocarcinomas
The AFP level in the serum of mice bearing teratocarcinoma was always low; serum titer with standard test-system for AFP never exceeded 1:2, and sometimes only a slight curvature of the precipitin line of the test-system was observed in agar precipitation, while with the serum of newborn mice titers of 1:1,000 or 1:2,000 were usually obtained.
With the immunofluorescence technique AFP was not revealed in the main area of the tumor sections. It was lacking in embryonal carcinoma, nervous, muscle, cartilage and connective tissues even in those cases when in these elements bright fluorescence specific for γG was observed. The latter protein served as a reference for non-physiological serum presence (see Engelhardt et al., 1971). Moreover, if several specimens of one tumor were taken, AFP was sometimes found in the sections of only a few of them, for example in one of three specimens taken from the first generation, in one of two specimens of the fourth generation, etc. AFP-containing structures usually occupied an insignificant portion of the sections and were clearly seen contrasting with dark background even when AFP level in the serum was quite low. Fluorescence in these areas was completely absent in consecutive serial sections incubated with anti-AFP antibodies neutralized with purified AFP preparation. γG was usually not observed in such structures.
Most intensive and frequent AFP-specific fluorescence was observed in the cytoplasm of epithelial cells of endodermal origin, lining cysts and tubes of various dimensions. Sometimes AFP-containing cells resembled those of visceral yolk sac as described by Pierce et al., 1959, 1960 (see Rugh, 1968, Fig. 1). In the section presented in Figure 1 there was no AFP in adjacent epithelium which was more intestinal in type (Fig. 1, f, g). In the same section AFP was present in several tubes and rosettes formed by cells which resembled those of the former AFP-positive epithelium. In many cases AFP-positive epithelium had no similarity with the epithelium of the visceral yolk sac (Fig. 2). Usually AFP-specific fluorescence was seen in the whole cytoplasm of cubic epithelium while in more differentiated prismatic epithelium it was restricted to the apex of the cells.
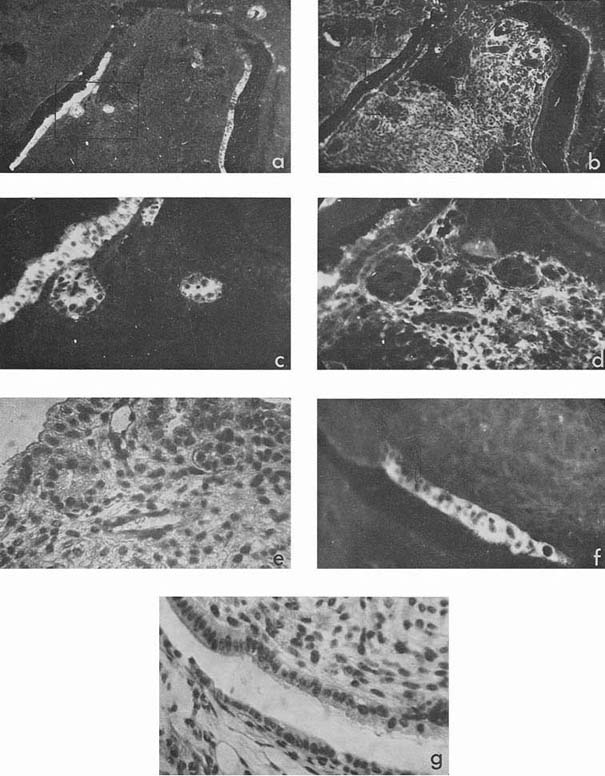 |
Figure 1
|
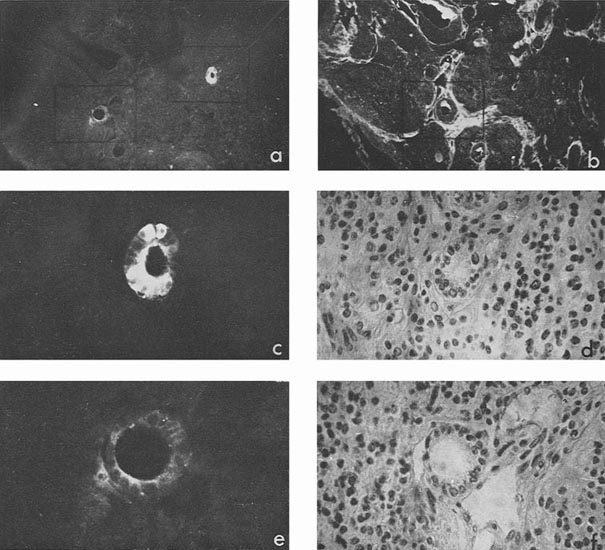 |
Figure 2
|
The intensity of fluorescence in such AFP-containing structures varied from very bright to almost background, and AFP was frequently detected in only a small proportion of cells of the lining.
Besides, sufficiently bright AFP fluorescence was episodically found in epithelial sheets, cell groups, and individual cells the origin of which we could not identify, although all of them were relatively well differentiated (Fig. 3 a, c). Luminescence of a still lower degree was found also in squamous epithelium arranged in keratin pearls. Pierce et al. (1960) supposed that such structures could possibly be derivatives of the visceral yolk sac.
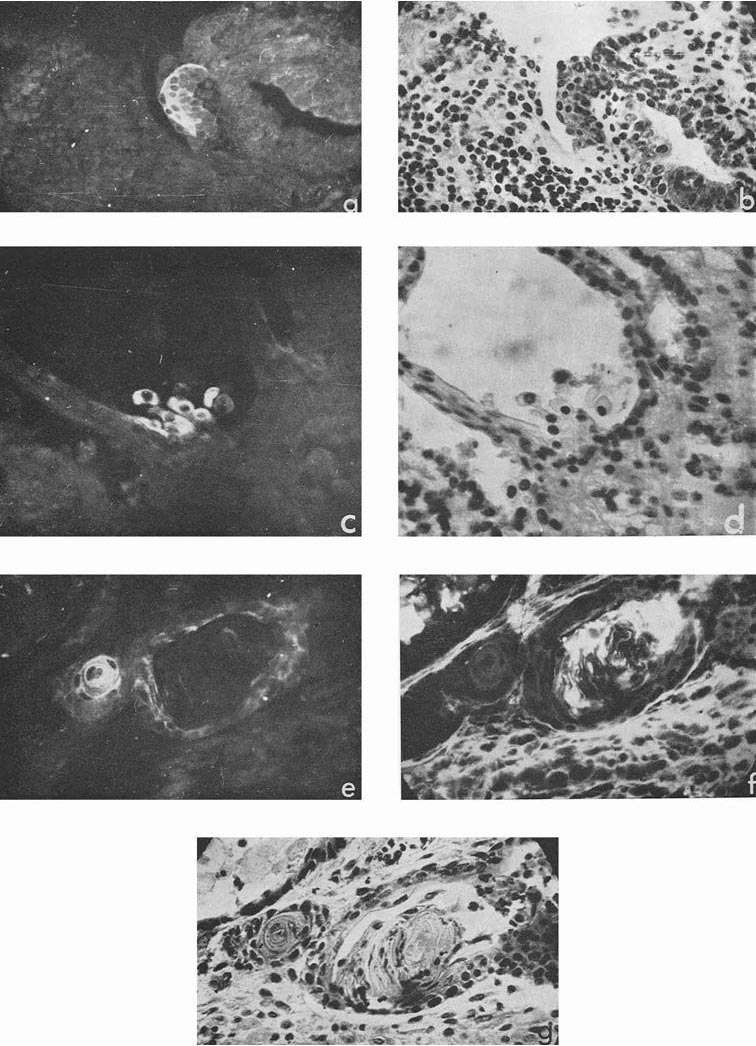 |
Figure 3
|
AFP in cysts and granules developing after intraperitoneal inoculation of teratocarcinoma
In four cases, when cysts were found in the ascites, AFP titers in the animal sera and ascitic fluids were 1:32, 1:8, 1:4 and 1:2, the difference being correlated to the number of cysts in the corresponding ascites. Morphologically, cysts were very similar to those described by Pierce et al. (1959, 1960); and their external epithelium was indeed very much like that of the visceral yolk sac (Fig. 4). Large and medium cysts with a diameter of 2-5 mm presented hollow transparent vesicles. Under the epithelial layer there was mesenchyme of the embryonal type, sometimes forming structures like primary blood vessels, as in normal yolk sac and agglomerations of embryonal carcinoma cells. In small cysts and granules fewer cavities could be found. Sometimes they contained only a central core of embryonal carcinoma cells and a peripheral layer of epithelium resembling visceral yolk sac. In other cases inside these bodies mesodermal and ectodermal elements could be also found. γG was present in AFP-containing cysts in insignificant amounts, on the surface of the epithelial layer, less frequently inside its individual cells, and, in trace quantities, in mesenchymal elements of the inner cavity. The degree of AFP-specific fluorescence in cysts was, as a whole, higher than in sections of solid tumors. The protein was regularly found in the cytoplasm of the outer layer of cysts. It should be noted, however, that AFP was usually not observed in all the cells of this layer, sometimes being evident only in a small proportion of cells and completely absent from the rest of them.
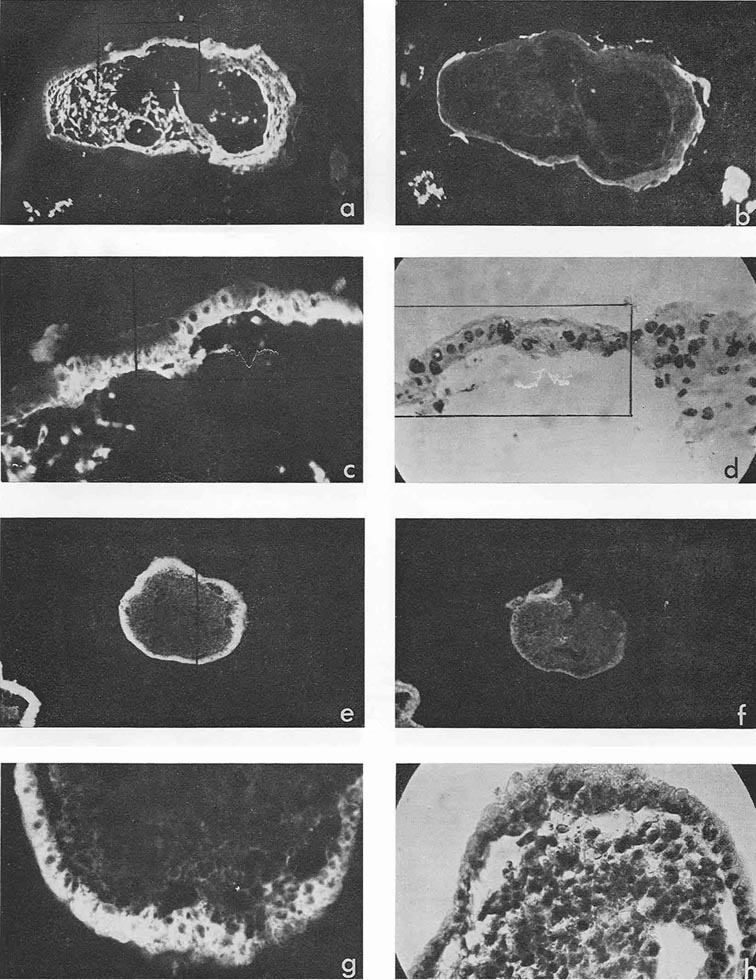 |
Figure 4
|
Mesenchymal elements, especially in medium-sized and large cysts, exhibited, as a rule, very bright AFP-specific fluorescence. Embryonal carcinoma cells usually did not contain AFP, but in two cysts bright fluorescence was observed in the inner cell mass after treatment with anti-AFP, in the absence of γG. It could be supposed that in cysts, unless they contained large quantities of γG, the test for the latter protein was not an adequate control to decide on passive accumulation of AFP. On the contrary, high AFP concentration in cyst medium could be the cause of its detection in mesenchymal and other internal structures of cysts.
When disc electrophoresis in polyacrylamide gel was performed with medium from two large cysts and pooled fluid from two smaller cysts, three main fractions were observed: albumin, AFP and transferrin, with only trace amounts of γG. AH the fractions have been identified by agar precipitation with standard test-systems for each protein (Fig. 5).
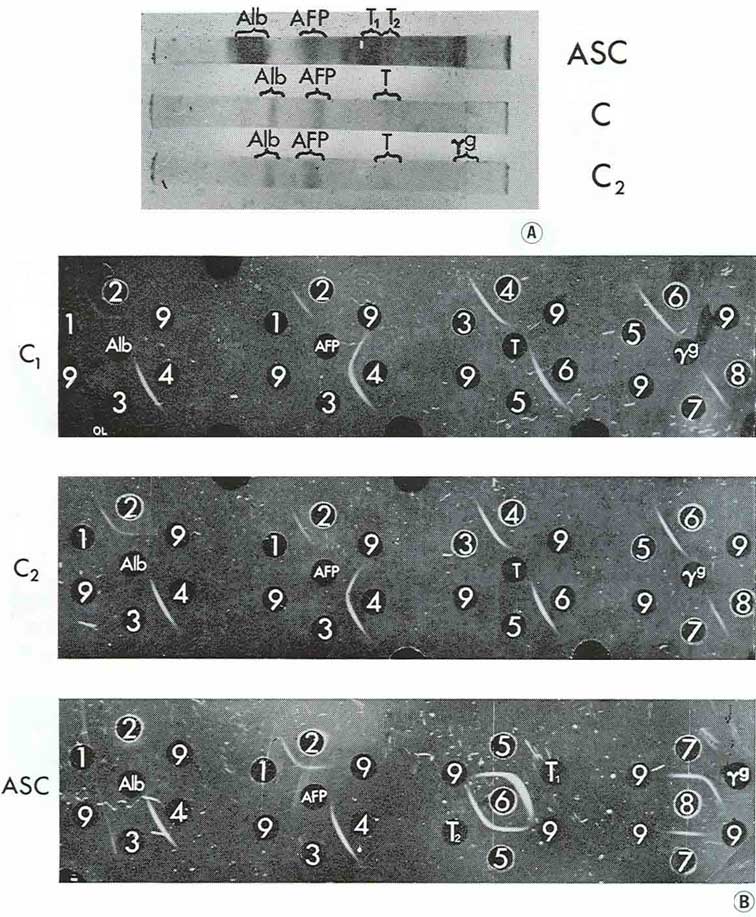 |
Figure 5
|
It should be emphasized that AFP was the principal component in cyst contents while in the corresponding ascitic fluids the relative concentrations of the proteins studied were inverse in quantity: AFP was present there in trace amounts and albumin, transferrin and γG were revealed in high concentrations.
Discussion
The fluorescence in the murine teratocarcinoma sections incubated with anti-AFP antibodies was specific for this very antigen: the antibodies were monospecific in agar-precipitation and were identified by the standard test-system for murine AFP. Also there was complete absence of fluorescence on the consecutive serial sections incubated with the same sample of anti-AFP antibodies neutralized with a nearly equivalent quantity of purified AFP.
As suggested previously (Engelhardt et al., 1971) we regarded as physiologically specific the presence of AFP only in those structures where γG was not revealed. The latter protein served as a marker for the passive presence of blood serum. It was noteworthy that AFP-positive cells were always differentiated. Sometimes they resembled cells of the visceral yolk sac but in most cases it was possible only to state their endodermal origin. The presence of AFP without γG was observed also in the mesenchyme of embryonal type inside the free-floating cysts.
The immunofluorescence technique provides information about the distribution but not about the exact sites of synthesis of the substances studied. However, in the case of AFP localization in murine teratocarcinoma it is very likely that AFP is synthetized in the cells of endodermal origin.
The AFP level in the serum of teratocarcinoma-bearing mice being always low, passive uptake from the serum is unlikely. The antigen was revealed by distinct fluorescence in a small number of structures where γG was completely absent. AFP-positive cells were very often found in the lining epithelium of tubes and cysts and sometimes bright fluorescence was observed in the secreted material inside the tubes.
There was positive correlation between the AFP level in the blood serum of the host and the frequency and intensity of AFP-specific fluorescence in the tumor sections, especially in the experiments with intraperitoneal injection of tumor suspension. The AFP concentration was significantly higher inside the cysts while the level of albumin and transferrin was much higher in the ascitic fluid.
Presence of AFP in the mesenchyme inside the cysts was most probably due to its adsorption from the cysts' medium, the more so as the protein was absent from the mesodermal derivates in the solid tumors. In this case estimation of γG presence could not be used because its concentration in the medium was negligible.
The direct proof of AFP synthesis by these cysts could be obtained in tracer experiments with isolated bodies. In the same experiments the origin of albumin and transferrin in the cysts' medium could be studied. As was demonstrated by Gitlin and Pericelli (1970) AFP, albumin and transferrin were synthetized in the human yolk sac. It would be important to know whether in teratocarcinoma structures analogous to the yolk sac retain the original pattern of synthetic activity and to use this model for studying the relations of synthesis of embryo-specific and adult serum proteins.
Our results are consistent with earlier findings of Mawas et al. (1969) obtained on clinical material. These authors failed to find AFP in the patient's blood serum after chemotherapy but observed it in the medium from a cyst inside the tumor and localized in some cells of pseudo-stratified epithelium lining the cyst.
In the experiments with murine teratocarcinomas, Kahan and Levine (1971) determined AFP by the complement-fixation test. They found the antigen in the cultural fluid of a clone growing in vitro as a monolayer of non-differentiated epithelial cells and after in vivo inoculation producing a tumor which consisted mainly of embryonal carcinoma cells and parietal yolk sac. AFP was not found in the serum of mice with primary teratocarcinomas and transplantable carcinomas of parietal yolk sac. We do not consider our data as contradicting these findings because of the different techniques used. It would be interesting to reinvestigate the same material by immunofluorescence with appropriate morphological controls.
It is highly probable that AFP-synthesis is a specific function of certain forms of normal early endodermal differentiation. Gitlin and Boesman (1966) were the first to find that AFP synthesis in entogenesis occurred not only in the embryonic liver but also in the yolk sac. Quite recently Gitlin et al. (1972) reported that besides these organs much lower but still quite definite AFP-synthesis was found in the gastrointestinal tract of the human fetus.
Different somatic tissues of murine teratocarcinomas very often correspond morphologically to the tissues originating at various stages of normal ontogenesis (Stevens, 1959) and they appear to be benign (Pierce et al., 1959, 1960). If the sites of AFP localization in murine teratocarcinomas are really the sites of its synthesis it is most probable that AFP appears in those cases when tumors contain differentiated cells analogous to the cells producing the protein during normal ontogenesis.
To test this suggestion a thorough study of AFP-synthesis and localization both in teratocarcinomas and hepatomas and embryos is needed. The published data about AFP localization in liver and yolk sac of rat embryo (Gitlin et al., 1967), in human fetal liver and placenta (Linder and Seppala, 1968) and in mouse fetal liver (Engelhardt et al., 1971) are still insufficient. Preliminary experiments on AFP localization in 6- to 6½-day-old murine embryos have been performed in cooperation with Dr. A. I. Goussev of this Institute. The traces of AFP were found at these stages in some cells of proximal endoderm while the fluorescence was absent in distal endoderm, mesoderm and ectoderm.
Similar studies on other embryo-specific proteins seem to be important for the analysis of differentiation in various tissues and for the understanding of the regularity of appearance of such proteins in malignant tumors.
Acknoledgements
We thank Prof. G. I. Abelev for his guidance and encouragement during all this work. We are grateful to Dr. L. Stevens of the Jackson Laboratory, Bar Harbor, Maine, USA, for strains of teratocarcinoma and mice. Our thanks are also due to Prof. A. P. Diban, of the Leningrad Institute of Experimental Medicine, Prof. A. Ja. Fridenstein of the N. F. Gamaleya Institute and Prof. T. A. Detlaf of the Institute of Developmental Biology, Moscow, for their valuable advice and help in identification of the AFP-containing structures. The investigation was partially supported by the World Health Organization and the International Agency for Research on Cancer.
LOCALISATION DE L'ALPHA-FOETOPROTEINE
DANS LES TERATOCARCINOMES MURINS TRANSPLANTABLES
L'élément sérique spécifique de l'embryon appelé α-foetoproteine (AFP) a été localisé par la technique d'immunofluorescence indirecte sur des coupes paraffinées de tératocarcinomes transplantables de la souche de souris 129/SV SL CP. Nous avons trouvé de I'AFP dans plusieurs types de structures occupant une partie insignifiante de la coupe totale. Nous avons constaté que la fluorescence specifique de I'AFP était particulièrement frequente et brillante dans l'épithélium d'origine endodermique qui entoure les kystes et les petits tubes; en outre, nous avons trouvé de I'AFP dans des couches ou des groupes de cellules ou dans des cellules individuelles d'origine non identifiée et dans l'épithélium squameux disposé en perles. Dans les kystes et les granules isolés du fluide ascitique, qui se sont développés après une injection intrapéritonéal de suspension de tératocarcinome, nous avons trouvé de I'AFP dans les cellules de la couche extérieure, qui sont très semblables à celles de la membrane viscérale, et dans le mésenchyme de type embryonnaire, à I'intérieur des kystes. Par électrophorèse analytique en gel de polyacrylamide, nous avons constaté que les kystes contenaient de I'albumine, de I'AFP, de la transferrine et de la gamma-globuline, et que la proportion relative d'AFP est sensiblement plus élevée que dans le fluide ascitique, par rapport aux autres protéines que nous avons mentionnées. Nous estimons que la localisation de I'AFP dans les tératocarcinomes correspond à des points typiques de sa synthèse dans I'embryon et que la synthèse de I'AFP est une fonction spécifique de certaines formes de différenciation endodermique.
References
- Abelev, G. I., Antigenic structure of chemically-induced hepatomas. Progr. exp. Tumor Res., 7, 104-157 (1965).
- Abelev, G. I., Alpha-fetoprotein in ontogenesis and its association with malignant tumors. Adv. Cancer Res., 14, 295-358 (1971).
- Abelev, G. I., and Bakirov, R. D., Synthesis of embryonal serum antigens by liver in vitro. Vopr. med. chimii, 13, 378-383 (1967) (In Russian).
- Abelev, G. I., Perova, S. D., Khramkova, N. I., Postnikova, Z. A., and Irlin, I. S., Production of embryonal α-globulin by transplantable mouse hepatomas. Transplantation, 1, 174-180 (1963). In Russian
- [Abelev, G. I. et al., Int. J. Cancer, 2, 551-558 (1967)]
- Davies, B. J., Disk-electrophoresis. II. Method and application to human serum proteins. Ann. N. Y. Acad. Sci., 111, 404-420 (1964).
- Engelhardt, N. V., Goussev, A. I., Shipova, L. Ja., and Abelev, G. I., Immunofluorescent study of alpha-foetoprotein (afp) in liver and liver tumors. I. Technique of afp localization in the tissue sections. Int. J. Cancer, 7, 198-206 (1971).
- Gitlin, D., and Boesman, M., Sites of serum α-fetoprotein synthesis in the human and in the rat. J. clin. Invest., 45, 1826-1838 (1966).
- Gitlin, D., Kitzes, J., and Boesman, M., Cellular distribution of serum α-fetoprotein in organs of the foetal rat. Nature (Lond.), 215, 510-534 (1967).
- Gitlin, D., and Perricelli, A., Synthesis of serum albumin, prealbumin, α-foetoprotein, arantitrypsin and transferrin by the human yolk sac. Nature (Lond.), 228, 996-997 (1970).
- Gitlin, D., Pericelli, A., and Gitlin, G., Synthesis of α-Fetoprotein by liver, yolk sac and gastrointestinal tract of the human conceptus.. Cancer Res., 32, 979-982 (1972).
- Goussev, A. I., Preparative set for separation of the substances by disk-electrophoresis in polyacrylamid gel. Biull. eksp. Biol. Med., 12, 104-108 (1969) (In Russian).
- Goussev, A. I., and Yazova, A. K., Isolation and purification of embryospecific α-globulins of humans and animals using preparative disk-electrophoresis in polyacrylamid gel. Biochimia, 35, 172-181 (1970) (In Russian).
- Kahan, B., and Levine, L., The occurence of a serum fetal α1-protein in developing mice and murine hepatomas and teratomas. Cancer Res., 31, 930-936 (1971).
- Kleinsmith, L., and Pierce, G. B., Multipotentiality of single embryonal carcinoma cell. Cancer Res., 24, 1544-1552 (1964).
- Linder, E., and Seppälä, M., Localization of α-foetoprotein in the human foetus and placenta. Acta path. microbiol. scand. 73, 565-571 (1968).
- Mawas, C, Buffe, D., Lemerle, J., Schweisguth, O., and Burtin, P., Recherche immunologique de l'α-foeto protéine ou fétuine dans les tumeurs primitives du foie et les teratomes malins de I'enfant (à propos de 18 cas positifs). Arch. Trans. Ped., 26, 779-790 (1969).
- Pierce, G. B., Dixon, F. J., Testicular teratomas. I. Demonstration of teratogenesis by metamorphosis of multipotential cells. Cancer, 12, 573-583 (1959).
- Pierce, G. B., Dixon, F. J., and Verney, E. I., Teratogenic and tissue forming potentials of the cell types comprising neoplastic embryoid bodies. Lab. Invest., 9, 583-602 (1960).
- Ruoh, R., The mouse. Its reproduction and development. Burgess Publishing Company, Minneapolis, Minn. (1968).
- Stevens, L. C, Studies on transplantable testicular teratomas of strain 129 mice. J. nat. Cancer Inst., 20, 1257-1275 (1958).
- Stevens, L. C, Embryology of testicular teratomas in strain 129 mice. J. nat. Cancer Inst., 23, 1249-1296 (1959).
- Stevens, L. C, and Hummel, K. P., A description of spontaneous congenital testicular teratomas in strain 129 mice. J. nat. Cancer Inst., 18, 719-748 (1957).
- Wise, R., and Oliver, J., Sites of synthesis of plasma proteins in the foetal rat. Biochem J., 100, 330-333 (1966).