
| Home page (in Russian) | Texts in English | Guestbook |

Pauline M.H. Mazumdar, editor. The University of Toronto. WALL & THOMPSON, Toronto
Preface
Introduction
Working Out of the Theory
Pauline M. H. Mazumdar
Institute for the History & Philosophy of Science & Technology, University of Toronto, Toronto, Canada
Prologue
The Template Theory of Antibody Formation and the Chemical Synthesis of the Twenties
Pauline M. H. Mazumdar
Institute for the History & Philosophy of Science & Technology, University of Toronto, Toronto, Canada
Chapter I
The Conception and Birth of Burnet's Clonal Selection Theory
G. L. Ada
Department of Microbiology, John Curtin School of Medical Research, Australian National University, Canberra, Australia
Chapter II
The Coming of Age of the Clonal Selection Theory
G. J. V. Nossal
The Walter and Eliza Hall Institute of Medical Research, Royal Melbourne Hospital, Victoria, Australia
Chapter III
Down-Regulation and Tolerance: The Trail from the Past
Bernhard Cinader
Department of Medical Genetics, University of Toronto, Toronto, Canada
Chapter IV
Is This Theory Necessary?
David W. Talmage
Webb-Waring Lung Institute and Department of Microbiology & Immunology, University of Colorado Health Sciences Centre, Denver, USA
Chapter V
Metaphors in Immunology
Fred Karush
University of Pennsylvania, Philadelphia, USA
Chapter VI
The Contribution of Synthetic Antigens to Our Understanding of the Immune Response
Michael Sela
Department of Chemical Immunology, Weizmann Institute of Science, Rehovot, Israel, and Department of Medicine, Tufts University School of Medicine and the New England Medical Center Hospitals, Boston, USA
Chapter VII
Some Reminiscences on the Contributions of Cancer Research to Immunology in the 1950s
Michael Potter
Chief, Laboratory of Genetics, National Cancer Institute, Department of Health and Human Services, USA
Chapter VIII
Two Decades of the Antibody Response: 1950–1970
Abram B. Stavitsky
Department of Molecular Biology & Microbiology, Case Western Reserve School of Medicine, Cleveland, USA
Chapter IX
Interaction Between Immunology and Genetics – Blood Group Systems as Important Early Models and as Tools
Rune Grubb
University of Lund, Department of Medical Microbiology, Lund, Sweden
Chapter X
Cell Mediated Immunity
Byron H. Waksman
Vice President, Research, National Multiple Sclerosis Society; Adjunct Professor Biology & Pathology, Yale University
Chapter XI
The Discovery of Thyroid Autoimmunity
Ivan M. Roitt
Department of Immunology, Middlesex Hospital Medical School, London, England
Chapter XII
Models for Autoimmunity
William O. Weigle
Department of Immunology, Research Institute of Scripps Clinic, La Jolla, California
Chapter XIII
The Development of Immunosuppressive Agents from X-rays to Cyclosporin
Hartmann Stahelin
Preclinical Research, Sandoz Ltd., Switzerland
Chapter XIV
The Relationship Between the Immune System and the Nervous System: Old and New Strategies
Branislav D. Jankovii
Immunology Research Center, Belgrade, Yugoslavia
Chapter XV
The Early History of Soluble Factors
H. Sherwood Lawrence
Infectious Diseases and Immunology Division, Department of Medicine, New York University Medical Center, New York, USA.
Chapter XVI
SRS-A Leukotrienes: From Definition to Biologic Implications
K.Frank Austen
Harvard Medical School
Chapter XVII
Immunodiplomacy: The Story of the World Health Organization's Immunology Research Programme, 1961–1975>
Howard C. Goodman
Professor Emeritus, Department of Immunology & Infectious Disease, Johns Hopkins School of Hygiene & Public Health, Baltimore, USA
Chapter XVIII
On the Origin of Cancer Immuno-Diagnostics
G.I. Abelev
Cancer Research Centre, Moscow, U.S.S.R.
Epilogue
Immunology Old and New: the Beginning and the End
Anne-Mark Moulin
Centre National de la Recherche Scientifique, Institut d'Histoire des Sciences, Paris; Affiliate to the Center for the Philosophy and History of Science, Boston University and to the Department of the History of Science, Harvard University
Index
Cancer Research Centre, Moscow, U.S.S.R.
This paper is intended to trace back some of the basic principles constituting the contemporary immunodiagnostics of cancer. First of all, we must formulate the present paradigm in this field, to serve as the starting point for our retrospective analysis, going back to the sources of each critical affirmation of the paradigm.
The basic assumption in this field is the belief that each tumour develops clonally from one precursor cell, that this cell is always less differentiated than a mature cell of that kind, and that the tumour retains the direction and differentiation stage of its precursor. The tumour produces antigens peculiar to the early stages of differentiation, the so-called oncodevelopmental antigens. It also possesses the differentiation antigens that are typical of the mature or maturing cells of its kind. If the precursor cell belongs to a family of clonally heterogeneous cells with slightly different antigenic properties, then its malignant counterparts will be identifiable by a strictly homogeneous antigenic marker.

Boris Brondz (left) , Howard Goodman (center), and Garri Abelev (right),
Cancer Research Center, Moscow, 1978
These three features, the re-expression of embryonal antigens, the presence of the differentiation markers, and the monoclonal nature of the group of cells, are widely employed in different areas of cancer immunodiagnosis. Other markers, due to the malignancy itself, are of less use in diagnosis: they include virus or virus-induced antigens in virus-associated tumours, or the protein products of cellular oncogenes.
Tumour antigens can also be divided into intracellular, membrane and extracelluar markers, which are used in histological, cytological and serological analyses respectively. Serological markers are widely used in both diagnosis and therapy monitoring, since their levels in the serum reflect the dynamics of the tumour mass in the body. Membrane markers are the main tool for precise classification of acute leukaemias according to stage-specific and differentiation antigens. They can also be used for identification of neoplastic cells in various body fluids. And the intracellular markers, for example, tissue-specific sets of cytokeratins, are very useful for identifying an unknown primary tumour from the characteristics of its metastases.
At present, immunological investigations enter routinely into clinical work, and are used to deal with an increasingly large number of forms of cancer. This vast and already respectable branch of immunology has originated quite recently, no more than twenty five years ago. I would like here to outline some of the points of departure of this discipline, discussing in more detail the events in which we were either immediate participants, or close and interested observers.
The discovery of the first carcino-foetal antigen, alpha-feto-protein, suggested a new and more or less universal principle for cancer immunodiagnosis. This discovery showed that hepato-cellular carcinoma, a common tumour which was very difficult to detect, had its own serological marker, and that this might indicate a new general approach in the search for tumour markers.
There was enough data at that time to suggest that tumours might possess embryo-specific antigens; Conheim had proposed that tumours originated from embryonal rudiments. Nevertheless, the finding of alpha-feto-protein production by liver tumours was quite unexpected. I have already written about how it happened (1), so I shall leave out some of the details here.
An embryo-specific glycoprotein, now called fetuin, was first demonstrated in the foetal calf by Pedersen in 1944 (2); then Bergstrand and Czar in 1957 (3) found a similar embryo-specific component in human serum. The foetal protein that they described was exactly like alpha-feto-protein; it had the same position in the electrophoretic spectrum and the same dynamics in development, although that was not discovered till later. The same protein was also discovered by Muralt and Roulet in 1961 (4) in human foetal sera, and by Pantelouris and Hale in 1962 (5) in the foetal mouse. However, no-one thought then of associating these proteins with tumours.
On the other hand, in our investigation of liver tumour, we found an antigen which was absent from normal liver, blood and other organs of healthy adult mice, as well as from tumours of other organs. According to these criteria, we considered it to be a tumour antigen in the strict sense, and named it hepatoma antigen.
The story of the finding of hepatoma antigen is as follows. In 1944, the outstanding Russian virologist and immunologist, Lev Zilber, founded the virology laboratory of the N.F. Gamaleia Institute for Epidemiology and Microbiology in Moscow. A few years before, Zilber had discovered the virus of Far-Eastern tick-borne encephalitis. The main trend in the laboratory investigations became the search for oncogenic viruses, or their protein constituents, which behave as tumour specific antigens.
The history of Zilber's seeking and striving for an approach to cancer virology and immunology has already been written. (6) As part of this research, Zilber and his group in 1949 (7) advanced a new and universal tool for the detection of tumour-specific antigens�anaphylaxis and desensitization. The reaction was applied to a great number of tumours of various kinds, and as a rule, with good results (8). When I joined Zilber's laboratory in the early fifties, soon after graduating from Moscow University, I started work on the nature of these tumour antigens.
The anaphylaxis-desensitization test that we were using is only a qualitative test, and it lacks internal resolving power. It was very difficult to use it to identify individual antigens in a complex mixture. However, immunodiffusion (first described by Ouchterlony in 1953) (9) and Immunoelectrophoresis (Grabar and Williams in 1953) (10) had just recently become available. I learned about the technique of precipitation in gel from two short papers by Bertil and Viveka Bjorklund (11). I was tremendously impressed with the new and quite surprising possibilities of these techniques. They were exactly what was needed for our problems. Both techniques resolved the products of reaction for each antigen-antibody pair, presented them in visible form, and permitted us to compare individual antigens without having to isolate them from complex natural mixtures. There had been nothing like this in the analytical immunochemistry of those days, and the new horizons thus opened inspired enthusiasm in everybody working in that field.
At first we were afraid that such a "crude and insensitive" reaction, as we thought, would not be able to reveal the subtle antigenic peculiarities which are inherent in tumour tissues in comparison with normal ones. Too few precipitin Unes had been seen by the Bjorklunds in their tissue extracts – too few! But it was impossible to resist the temptation to work with such an extraordinary reaction and we started ... We started after long and timid attempts, at first with our colleague Dr V.A. Parnes, on a human leukaemia system then with another colleague, Dr Z.A. Avenirova, on a mouse liver-hepatoma system. In both studies, we cooperated with Dr. Z.L. Baydakova, who prepared strong and avid precipitating human sera for us. Our goal in all these investigations was the same: to find out whether immunodiffusion was adequate for the comparison of tumours with their normal counterparts.
All was hard and slow in this work: the production of strong antisera, the preparation of purified agar, making the templates, photographing the results. The main difficulty was our lack of experience in the philosophy, so to speak, of gel-precipitation.
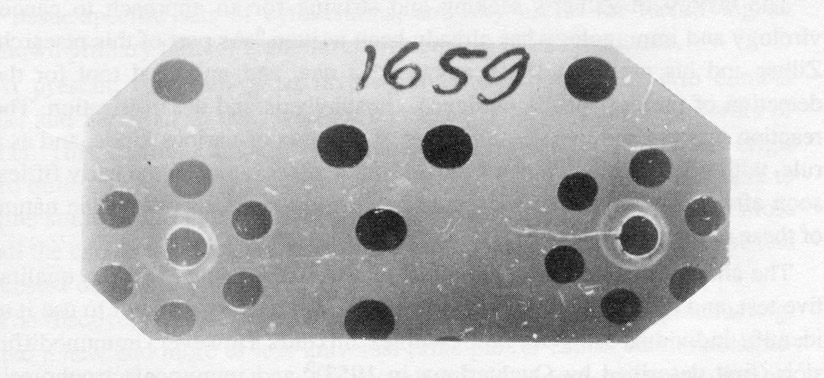
Experiment of February 7, 1962:
The first finding of "Hepatoma Antigen" in mouse embryo.
In the central wells of seven-well figures – anti-hepatoma serum, absorbed by normal liver antigens (adult);
in the upper wells – hepatoma extract in optimal dilution;
Clockwise – double dilutions of embryonal liver extract (from 1/8 to 1/128), in left figure,
and extract of whole embryo (from 1/8 to 1/128), in right figure.
Large wells are filled with saline
The first precipitation lines were obtained the leukaemia system but that work, unfortunately, did not progress far enough (12). The liver-hepatoma studies, on the other hand, gathered the speed of a race. Soon, in 1957, our group was reinforced by Ms Nataliya Engelhardt, who joined us just after graduating from the university.
To our great astonishment, we found that a family of liver-specific antigens were lost in our hepatoma strain (13). It was a real and surprising success, and we were ready to go completely into the new and fascinating field of antigenic simplification which was opened to us by our first findings. We would have been quite satisfied to have continued to work along these lines, but here Zilber displayed his energy and will-power: he demanded and insisted that we focus all our attention on the search for a hepatoma-specific antigen.
This turned out to be a difficult task. We compared crude fractions of cytoplasmic granules from liver and hepatoma, where a very faint line of hepatoma antigen glimmered against the strong background of liver antigens. It was difficult to get it surely and reproducibly in the spectrum of precipitating lines. Finally, with some changes in extract preparation and test arrangement, it became possible to do this.
First, the amount of this antigen – we called it AH, antigen of hepatoma – was higher in simple saline extract from the tumour, than in its cytoplasmic granular fraction, with which we were operating. Then we changed the arrangement of the wells in the gel precipitation to the so-called square pattern, to clarify the most important area on the plate, where AH crosses the lines of liver antigens (14). That arrangement was widely accepted later on in immuno-chemistry, and we have continued using it up to the present.
In July 1958, Zilber demonstrated the specific hepatoma antigen, as revealed by immunodiffusion at the VII International Cancer Congress in London (15). Thus gel precipitation gave us much more than had been expected. We have now been relying on it almost exclusively for more than a quarter of a century. It has suggested our schedules of analysis, and, so to say, our philosophy of investigation at the level of the individual antigens (16). But seeing the line of AH in gel was only the start for further studies. We had to isolate the AH from the complex mixture, to get mono-specific antibodies to it, and we could learn much more about it.
We were engrossed by this goal entirely, all the more so that immunodiffusion and Immunoelectrophoresis allowed us to work with open eyes; the techniques themselves suggested the approaches to the isolation and purification of AH. At once we started together with Vladimir S. Tsvetkov to develop an immunochemical method for AH purification, which we called "immunofiltration." Roughly purified AH with the antigenic admixtures inseparable from it by agar gel electrophoresis, was allowed to migrate electrophoretically into an agar block, where it met in its path the IgG from a serum raised against normal liver. AH had the mobility of a serum alpha-globulin and migrated to the anode, while the IgG was transferred by the electro-osmotic flow to the cathode. When antigens and the antibody filter met in the agar block, the normal antigens common to liver and hepatoma were precipitated or slowed down, while the AH continued to move on past them. In that way, AH was separated from the antigens common the normal liver, and it could be obtained in pure form (17). It was the first isolated tumour-specific antigen, and we were naturally proud to have it in hand.
In parallel work with Avenirova, we were able to obtain purified antibodies to AH. They were eluted by incomplete dissociation of the AH-anti-AH precipitate in an acid medium (18). Thus we now had an ideal system-purified pair of AH and anti-AH, both active in gel precipitation. Immunodiffusion had exceeded all expectations!
I was sure at the time that we were working outside the mainstream, in a kind of niche. So I was astonished when looking through the Annual Reviews of Biochemistry for 1962 to see a thorough description of our work, which had been published mainly in Russian, by Eugene Day in 1962 (19). Later on Day issued a very original book on cancer immunochemistry (20), with a lot of historically interesting material in it.
At this point we were confronted with some new problems, which led us, if not into an impasse, at least into an area of skepticism and doubt. What was the origin of AH? We had not found it in all hepatoma strains; it was absent from some primary hepatomas which had been induced by carcinogens. Perhaps it was due to an accidental virus passenger; perhaps it came from cholangiocytes, not from liver cells: the characteristics would fit either supposition. What we needed was an immunofluorescence test that would identify AH in normal tissues. In 1961-1962 we were involved in these problems, full of anxiety and doubts. Ms Engelhardt tried immunofluorescence, but failed; her failure was not only due to the lack of a fluorescence microscope, reagents and experience, but also to the inscrutable nature of AH. Only in 1968-1969 did she succeed in localizing the antigen for the first time (21). Meanwhile, we had improved the sensitivity of the gel diffusion test about 10 times by the introduction of the immunofiltration technique mentioned above.
Surprisingly, the natural course of the work was now interrupted and its direction changed. Working with Ms Ninel Khramkova, a young colleague from our group, I continued with a certain amount of success to investigate the problem of antigenic simplification in hepatomas (22). We found that the antigenic pattern of different hepatomas was unique for each strain. We decided to compare the patterns peculiar to different hepatomas with the antigenic patterns of the liver in different stages of its development. The test system on AH was included in the study without any particular expectations. The very first result was so unexpected that it was difficult to believe. Embryonic liver extract contained so much AH that it smashed the precipitin line from antigen excess. We had no such concentrated AH in our collection, so there could not be a labelling mistake! Soon it became clear that AH is not a tissue antigen but a serum component, an embryo specific alpha-globulin, which simply contaminated all the embryonic organs from which the blood had not been completely washed out. It was not only present in embryo blood, but also in the blood of hepatoma-bearing mice as well. We felt ourselves extremely uneasy. Might it not be simply a so-called acute-phase protein, produced by the host liver, and contaminating the tumour and the normal organs of the tumour-bearing animal? Such things were already known in rats (23).
Fortunately, the story of AH had a happier ending. I have written about it elsewhere in 1983 so I shall simply say that after four months of hard work and further vicissitudes of hope and fear, we could finally assert that: a) AH is an embryonal serum alpha-globulin, absent from the adult mouse, b) It is probably produced by the embryonic liver, ceasing as it matures, c) Its syn thesis is resumed in some, though not all, relatively undifferentiated hepatomas. Some primary hepatomas also produce alpha-F globulin, though more rarely than the transplantable ones. More recent work has suggested that there is a genetic component involved in this, d) The last, most important affirmation that we were able to make was by means of hetero-transplantation of the mouse tumours into rats treated with cortisone, in whose serum we were able to detect mouse alpha-F globulin. Finally, we saw a wave of alpha-F globulin production in partially-hepatectomised mice that presumably accompanied liver regeneration.
All these results had been obtained by May 1962, and they were included in my report to the VIII International Cancer Congress which fortunately just then was held in Moscow. I had been invited to the Congress some months before, to take part in a discussion chaired by Pierre Grabar of the Institut Pasteur. Grabar had visited Moscow in 1961, so he already knew of our work on AH; he himself was interested in the application of immunoelectrophoresis to the detection of tumour and tissue-specific antigens (24). Chester Southam of the Sloan-Kettering Institute also knew of our work; he came to our laboratory before the Congress, and helped me to translate my Congress report into English.
During the Congress, we got to know a number of foreign scientists in tumour immunology. We met Karl and Ingrid Hellstrom, and Hans Sjogren and Britta Slettemark-Wahren from George Klein's laboratory in Stockholm, Robert Baldwin from the British Imperial Cancer Research Fund, Arnold Reif from Tufts University in the U.S.A., and many others. Herbert Morgan, from Rochester University in New York, a very vivid person who had previously visited Zilber's laboratory, tried to put us in touch with as many foreign scientists as possible. It was a lovely time; there was a rising wave of avid and benevolent interest by western scientists in our progress. Many of them visited our laboratory, and we established friendly and quite open relations with them which have continued for many years now. I am writing that not only to remember thankfully our foreign colleagues, but also to note how quickly the data on alpha-F became known to tumour immunologists. I think that our data were accepted at that time as a paradigm of tumour specific antigens.
We had not considered the possibilities of using alpha-F for the clinical diagnosis of hepatomas in any of our publications (25), though only one step separated us from being able to do it. We needed an antiserum to human foetal serum, absorbed with an adult serum, and tested against hepatoma patient serum. But we were not ready to take this step. We were prevented from taking it by some "excess" knowledge: we knew that alpha-F was produced by regenerating liver, that not every strain of hepatoma produced it, and that nodules of primary liver cancer in mice rarely did so. We assumed that it was a product of normal as well as malignant liver cell proliferation. So this practical step was taken not by us, but by the Astrakhan biochemist Yuri Tatarinov.
At that time, Tatarinov had the chair of biochemistry at Astrakhan Medical School; he was working on the antigenic properties of serum proteins in various diseases. The starting point for this work had been an erroneous observation by one of our widely-known medical biochemists that seemed to show that the antigenic properties of the serum proteins are not constant, but show pathologic changes in different diseases. This work induced a whole series of studies in our country aimed at developing immunodiagnosis based on antigenic peculiarities in the serum proteins. Tatarinov's work was part of this. Dr. A.E. Gurvich, my colleague in Zilber's department, was the supervisor of Tatarinov's doctoral thesis, and he introduced us.
Tatarinov had not been able to confirm the observations on the antigenic changes of serum proteins; he gained our support because he brought common sense into a very popular but wrong direction of research. I remember discussing our data with Tatarinov which showed that alpha-F in normal serum is the same immunologically as the one produced by malignant hepatocytes in culture. There was no change in its specificity. In a few months, Tatarinov had obtained an antibody to human foetal serum, absorbed it with normal adult serum, and tested it against the serum of a patient with hepatocellular cancer. This patient revealed the same alpha-F that was present in normal foetal serum (26). The circle was closed. Tatarinov went on to complete a series of tests on hepatocellular carcinoma patients, patients with other types of liver and non-liver cancer, and on 200 normal subjects; 6 out of 6 of the hepatocellular cancer cases were positive, and none of the others (27).
I was still rather skeptical about the diagnostic data; I thought it was an overestimate. We knew only too well that all liver regeneration produced it, and that not all liver tumours did so. We did prepare a test-system for human alpha-F, but I felt that it was Tatarinov's field, and I held back my co-workers from an "invasion" of it. However, sometime in 1966, Dr. N.I. Perevodchikova, a clinician from the Institute of Experimental and Clinical Oncology in Moscow asked me to test the value of alpha-F in cancer diagnosis. I wanted to direct her to Tatarinov, but she insisted in working with us, so we became involved in the diagnostic study. Very soon, we had confirmed Tatarinov's results with large numbers of patients and controls of our own. Our results showed that about 70% of the hepatocellular carcinoma cases were positive; but we were very surprised to find clear positives in 13 out of 32 cases of teratocarcinoma of testis as well (28).
The next important step in the popularization of the alpha-F test was part of the International Collaborative Study organized by the Immunology Unit of WHO and the International Agency for Research on Cancer (IARC) (29). The history of this study goes back to the visit of Howard Goodman and Michael Sela to Moscow in July 1966. Goodman and Sela were at the Gamaleia Institute, and they visited our laboratory and discussed our problems with us. I asked Dr. Goodman if he would help us to get serum samples from South Africa, where primary liver cancer is very common. He was interested and said that he would help us, but on condition that I myself should go to the newly organised WHO centres in Africa to take part in the work and make direct contacts. This did not seem very realistic to me, and after Goodman left, I almost forgot about it. However, in the fall of 1966,1 got a letter from Dr. Gregory O'Conor, who headed the Epidemiology Department of IARC, which had just been established. Dr. O'Conor referred to Goodman's visit, and wrote that it might be possible to carry out our study along with another project that they were organising in Africa, and he invited me to come to Geneva for discussions. I answered that this could be done by mail. In February of 1967,1 got an excited letter from Dr. O'Conor saying that during his travels in Africa, he had found at Dakar University an actively working French group studying the alpha-F test in primary liver cancer, with very promising results. O'Conor asked me urgently to come to Geneva to start discussions as soon as possible.

Lev A. Zilber (right) and Pierre Grabar (left);
Photo taken in the early '60s.
The African study had been initiated by Grabar, who at that time was seriously involved in alpha-F work. One of Grabar's co-workers in the laboratory, Jose Uriel, was at that time supervising a doctoral student, Mark Stanislavski-Birenswaig. In 1965, Stanislavski obtained a full and very expressive picture of embryospecific antigens in embryo rat serum. He found three antigens, one of which, called LA, was obviously homologous to mouse alpha-F, and it reappeared in the serum of rats with primary carcinogen-induced liver tumours (30). We too had obtained similar results in rats, and we compared them during my visit to Grabar and Uriel in their laboratory in the winter of 1965. Grabar and Uriel had worked out a test system for human alpha-F, and had at once started trials with French colleagues in Dakar. Rene Massayeff, a biochemist, and Rene Camain, a patho-morphologist, were working at Dakar University, and they quickly collected a very large group of sera, from 55 primary liver cancer patients. They were able to demonstrate very clearly the high specificity and sensitivity of the alpha-F test (31). It was this project that O'Conor had come across during his travels through Africa. Very soon there followed an energetic cable from Goodman inviting myself and my director, Professor O.V. Baroyan, to take part in discussions on primary liver cancer. The invitation worked out surprisingly well, and soon Baroyan and I, Uriel, Grabar and Goodman, and O'Conor and his assistant Zdenek Trnka met in Geneva.
Goodman suggested that we make use of a number of WHO-IARC centres in Africa to collect and test sera from liver cancer patients. There were centres in Nigeria, the Congo (Kinshasa), Kenya, Uganda, the Ivory Coast and Senegal that we could use. But it was suggested that we should start the work by visiting these places to establish direct personal contacts with the local investigators; the local people must be equal participants in the experiment, and not just the source of the materials. Uriel said that he already had contacts in Dakar through Massayeff, and did not need any new ones. I asked Goodman to include Tatarinov in the project and in the trip to Africa, because it was he who had started to work with human patients, and being in Astrakhan, far from Moscow, he had fewer chances for international cooperation. The suggestion was accepted at once, and just a year later, together with Tatarinov, we set out for Africa.
Rene Masseyeff (left), Yuri Tatarinov (center) and Garri Abelev (right) in Dakar (Senegal),
in Masseyeff's Garden (March, 1968)
We brought with us all that was needed for the tests to be carried out in "field conditions": immune sera, antigens, templates for gel precipitation, agar, capillary tubes, etc,, as well as materials for lectures and discussions. We were also accompanied by Dr. Albert Tuyns, a man with great experience in international relations, who was charming, clever and tactful, and whose help was invaluable to us.
Our visits usually followed the same pattern. First we would meet with the head of the centre, then we gave a general lecture for the physicians and students of the University, followed by tests of the blind-labelled sera from the clinics, with a demonstration of all the technical details to all visitors. Sometimes it seemed like a kind of fakir performance. I vividly recollect the lecture in the big student hall of Abidjian University in the Ivory Coast. I delivered the lecture in my "English," Tuyns translated it into French, brightly-dressed black students put it down in their notebooks. On the day we arrived, we had met the Dean, who seemed rather cold and mistrustful; we suggested that we should demonstrate the reaction and test the blind-labelled sera from the clinic. At once the Dean offered to take blood from primary liver cancer patients along with several hard controls. Tatarinov and I started work, demonstrating the details of the technique at the same time to the laboratory staff. We were prepared to meet some alpha-F negative patients, some mistaken diagnoses, and what we were most afraid of, simple labelling mistakes, of the test-tubes or of the samples on our plates. The visits were very short, there was no time to repeat an experiment; in addition, the attitude in most places was rather skeptical, except in Dakar where they had their own positive experience.
Early in the morning, we were already in the laboratory. Six of the sera were clearly negative, three were very strongly positive, and one weak or doubtful. Only one physician knew the diagnoses, but he had not yet come to the clinic. During the lecture, someone brought a note to Tuyns. Probably the diagnoses, I thought. I went on talking, with Tuyns translating, about the results in Moscow and Astrakhan, and saying that yesterday we had performed the tests here. Tuyns put up the results and the audience started to applaud as the pluses and minuses appeared on the table. Complete coincidence of clinical and alpha-F data!
Naturally, it was easier to talk about future collaborative work after a demonstration like this. We also left our test-system at each of the centers, making no secret of anything and explaining all the details and peculiarities of the reaction. That was the most important thing. We established contacts with the African investigators in the best possible way, even with Massayeff and Camain, who were already working with Grabar's group. The friendships we made have lasted for many years.
The serum samples collected from the African centers were sent back to Paris, where they were divided into three parts, lyophilised and distributed blind-labelled to three laboratories, Uriel's in Paris, ours in Moscow and Tatarinov's in Astrakhan. The answers were sent independently to Lyon, where all the participants met in 1969 – from the African centres, from Singapore and Jamaica, from France, Geneva and the U.S.S.R. – to sum up the results of the cooperative study. The results were very satisfying; the tests showed high specificity, good agreement of independent determinations and low percentage of false positives. At the same time, the present state of the problem was discussed, and a unified nomenclature arrived at. From then on, alpha-F and its many synonyms would be known as alpha-fetoprotein.
The international experiment was one of the first IARC scientific programmes. Its significance was that, though it added no new data, it legitimated the AFP test, and gave it international approval, the seal of quality, so to speak. But most importantly, the experiment brought together almost all the people in the world who were involved in the problem. Simple, open and trustful relations were established in the "AFP community," against a background of enthusiasm. Gregory O'Conor and Howard Goodman made a valuable contribution to this work, not only by their professional experience, but even more by their personal charm and goodwill.
Interrupting the AFP story somewhere near the brink of contemporary studies, I shall allow myself the liberty of making some comments. What have we learned from it? First, that some of the most interesting results of a study are often completely surprising, unpredictable and quite apart from the original goal of the study. Second, that the data which may be disappointing within the framework of an accepted paradigm may be of principle importance in another one, which can be revealed and formulated through these data. Third, one must always have open eyes and ears to see and hear what the system under study would like to tell him, and not confine one's attention to the happenings that one was prepared to observe when arranging the experiment. And finally, new methods give us new eyes, discriminating events which had not been resolved before. The level of contemporary science is determined by the resolving power of contemporary methods.