
| Home page (in Russian) | Texts in English | Guestbook |
Transplant. Rev. (1974), Vol. 20
Published by Munksgaard, Copenhagen, Denmark
G. I. Abelev
Tumor Immunochemistry Laboratory,
N. F. Gamaleya Institute for Epidemiology and Microbiology,
Moscow
Reappearance of embryonic and fetal antigens in tumors is a rather common phenomenon (Alexander 1972, Anderson et al. 1972, Hirai & Miyaji 1973). Different mechanisms may be responsible for this �antigenic reversion� in different systems, but none of them has been elucidated yet. The alpha-fetoprotein (AFP) system seems to be most promising in this respect, since here we deal with a distinct elementary character, an individual and well-defined protein which dynamics in ontogenesis and pathologies have been extensively studied (for review see: Abelev 1971, Masseyeff 1972, Hirai & Miyaji 1973). Good opportunities for studying the nature of the phenomenon are provided by availability of several immunochemical and immunohistochemical methods for AFP determination as well as by versatile in vivo and in vitro models in which its production can be examined under strictly controlled conditions.

Some time ago, we proposed the hypothesis that AFP synthesis was accomplished by differentiated cells of the foetus as their specific function during early ontogenesis, and that the tumor stem cells of hepatoma and teratoblastoma maintained the ability for differentiations including those which lead to cells responsible for AFP synthesis in normal ontogenesis (Abelev 1968, 1971). From these positions it is of primary interest to investigate the cellular basis of AFP production, to find out which cell types are responsible for AFP synthesis under normal conditions and in tumors, and whether these cells constitute the ultimate result of differentiation or a transistory stage between the precursor cell and the mature differentiated form.
The necessity of solution on this point is evident, irrespective of theoretical assumptions of the investigator, since without it, neither a rational basis for diagnosis of corresponding tumors nor further analysis of the nature of the phenomenon are possible.
To consider the problem, evidence gained in different approaches to AFP can be used. Thus, quantitative data on AFP production during carcinogenesis and in established tumors can be related to histogenesis of tumors and cell population dynamics in precancerous period. Direct immunohistochemical approach, still little used, should help in identification and characterization of AFP-producing cells. And, finally, experimental analysis of liver and hepatoma cultures in vitro may help in determining relationships between AFP-producing cells and the clonogenic or colony-forming stem cells in normal and tumor tissues. The latter approach has not been used yet in studying the problem.
In the present article, we summarize results of investigations made in our laboratory during recent three years and make ah attempt, by considering them together with the data from the literature, to analyse the problem of cellular basis of AFP synthesis.
The article includes materials on recent developments in highly sensitive methods for AFP determination, on AFP production in germ cell tumors, and on AFP behavior during hepatocarcinogenesis ahd in hepatocellular cancer. The article considers both biological and clinical aspects of the problem. Our previous data have been exhaustively reviewed earlier (Abelev 1965, 1968, 1971).
Highly sensitive radioimmunodiffusion methods of AFP determination
The major part of experimental and clinical findings on AFP production by hepatomas and embryonic carcinomas were obtained by gel precipitation test and have mostly qualitative character. They give plenty of material on the frequency of AFP in certain pathologies, but events taking place in the 'subthreshold' zone of the test remained as a rule out of observation. Special attention has been paid therefore, to development of quantitative methods of high sensitivity which would permit following AFP at all levels exceeding the normal �background' and to get quantitative picture of its distribution and dynamics in various pathologies. Two groups of methods were employed for this purpose: radioimmunoassay (RIA) in its various modifications (Ruoslathi & Seppälä 1971, Purves 1972, Hirai et al. 1973) and radioimmunodiffusion (RI) tests (Rowe 1969, 1970, Abelev & Elgort 1970, Elgort & Abelev 1971).
RIA is the most sensitive among immunochemical methods and permits detection of as little AFP as 1 ng/ml. Its drawbacks are: the lack of resolving power, the requirement of highly purified antigens and antibodies for the test-system, the instability of heavely labelled antigens, and the complexity of the equipment used. From the very start of our work on this problem, we aimed, therefore, to construct sensitive techniques possessing resolving power, in other word, to raise sensitivity of immunodiffusion tests. For this purpose we used the RT principle developed by Rowe (1969, 1970) slightly modified by us (Abelev & Elgort 1970, Elgort & Abelev 1971). Two more techniques employing this principle have been developed recently (Abelev 1973, Abelev et al. 1974), in which the resolving power of immunodiffusion is combined with the sensitivity of RIA. Brief descriptions of the above developments are given below.
Radioimmunodiffusion method (RI)
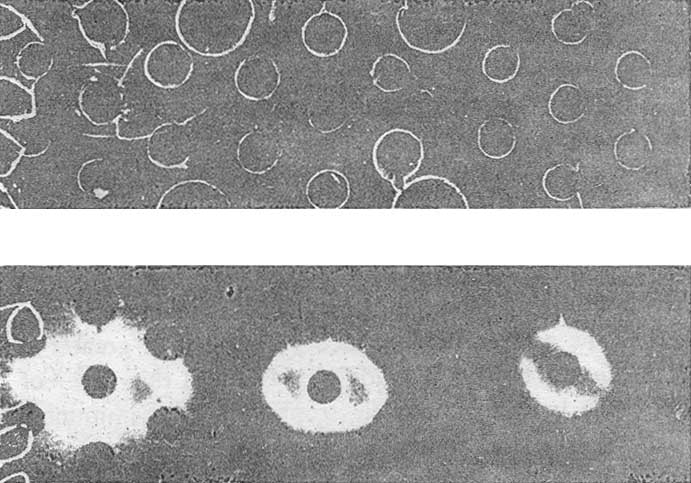
In our modification, AFP is determined by double diffusion in gel using monospecific anti-AFP antiserum and AFP-containing material as the reference antigen. The reagents are taken in equivalent proportions and diluted in parallel so that the precipitation line produced by them, though still clearcut, becomes progressively weaker. The sensitivity of determinations is known to increase directly with dilution of the test-system (Khramkova & Abelev 1961), and the faintest but still visible precipitation line assures detection of AFP levels equal to 1 or 2 µg/ml. This is assumed to be the unit. Reactions produced with this test-system upon further dilution 8-, 16-, and 32-fold give invisible precipitation lines which, however, can be revealed by I125 – or I131-labelled antibody towards IgG of the antiserum (Figure 1).
|
Figure 1. Detection of AFP by radioimmunodiffusion test. A – agar plate following immunodiffusion and staining with amidoblack; B – radioautograph of the same plate. 1 and 2 – antiserum and antigen, respectively, of the test-system for AFP; 3 and 4 – the test-system for AFP diluted 1:2; 5 and 6 – the test-system for AFP diluted 1:4. 7 and 8 – tested serum samples. 9 and 10 – saline. |
Thereby, immunodiffusion becomes about 30 times more sensitive without any loss in specificity. Further increase in sensitivity is achieved by preliminary 3 – to 4-fold concentration of material to be examined by lyphogel. Altogether, the sensitivity is raised to about 15 ng/ml AFP (Table I).
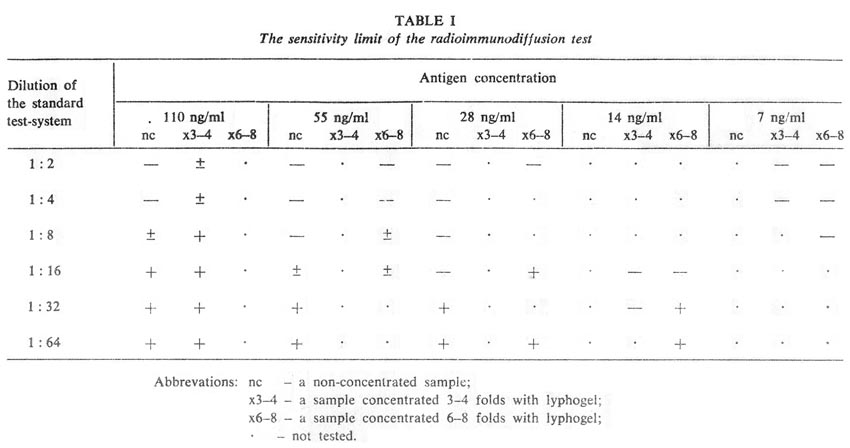
Results of the test are evaluated semi-quantitatively on the basis of the sensitivity of the test-system diluted to the limit of visibility (1-2 µg/ml). The range of AFP concentration in examined material is calculated as being between T/n.c and T/n.c µg/ml, where: T – the reciprocal titer of the examined antigen relative to the particular test-system used; n – the degree of dilution of the test-system (as a unit the test-system with sensitivity 1-2 µg/ml is taken); and c – the degree of preliminary concentration of the sample.
Details of our modification of the method were described earlier (Elgort & Abelev 1971), but several essential improvements making the technique more simple and quite safe in use have been given in a recent paper (Abelev et al. 1974).
With the RI test, greatly increased sensitivity is attained together with full specificity of immunodiffusion and quite stable and universal labelled reagents, the only strict requirement being monospecific anti-AFP antiserum. The sensitivity of RI test (~ 15 ng/ml) is sufficient for detecting 'pathological' levels of AFP in man and animals. However, this is one order of magnitude lower than with RIA, and this stimulated further work on RI test to make it as sensitive as RIA.
Electrophoresis-precipitation in polyacrylamide gel (EPAG)
(Abelev 1973, Abelev et al. 1974)
High sensitivity in this method is achieved by preliminary electrophoretic concentration of the antigen and by incorporating it in greater amount into the zone of reaction. The test is made in a flat and thin layer of polyacrylamide gel (PAAG) in which both electrophoresis and immunoprecipitation are carried out. The method consists of three stages. First, the antigen is concentrated about 30-fold by electrophoresis from high-porous (4 per cent) to low-porous (5,6 or 7 per cent) PAAG, using a discontinuous buffer system. The concentrated antigen is obtained as a long condensed zone in the low-porous PAAG (Figure 2). The concentrated antigen is then allowed to react, right in the low-porous gel, with a maximally diluted test-system for AFP.
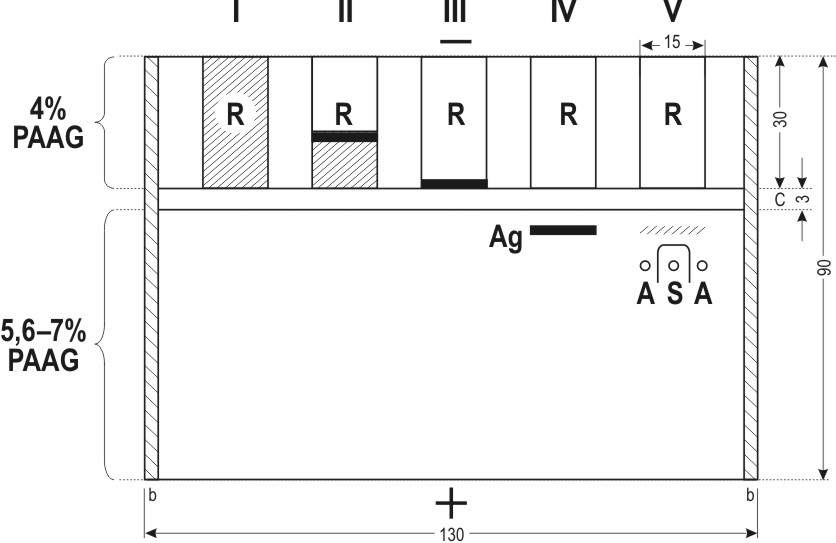
Figure 2. Schematic representation of the Electrophoresis-Precipitation-in-Polyacrylamide-Gel (EPAG) technique. R – reservoirs for the antigen. Successive stages of the test are shown: I to IV – concentration of the antigen and incorporation into the low-porous gel; V – detection of the antigen by immunoprecipitation in gel. Ag – antigen position following incorporation into the low-porous gel; A and S – antigen and antiserum, respectively, of the test-system for AFP. b – glass barriers; c –concentrating layer of 4 per cent PAAG. All dimensions are in mm. |
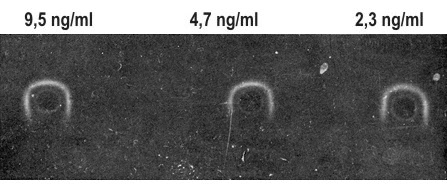
When the reaction is over, the plate is washed, treated with I125-labelled anti-IgG antiserum and subjected to radioautography (Figure 3).
|
Figure 3. A radioautograph of AFP detection by EPAG technique. The test-system is diluted 1:64. Samples contained from 9.5 to 2.3 ng/ml AFP. |
In this technique, the reaction zone gets about 100 times more antigen than usual, as a result of preliminary concentration and greater width of the antigen band. The additional gain is sensitivity, obtained by radioautography of invisible precipitation lines, is 30 –to 50-fold. Thus, sensitivity is raised from 2000 to 4000 times compared with ordinary gel precipitation. EPAG is capable of detecting AFP in concentration up to 0.5 ng/ml, that is with sensitivity similar to that of RFA (Table II). At the same time, the resolving power of double diffusion in gel is fully maintained, which is an advantage of the method.
To perform the test, one does not need highly purified antigen and antibody; only the antiserum should give a single precipitation line with the AFP-containing material. For radioautographic detection of precipitation bands, we use antibodies to rabbit IgG moderately labelled with I125 according to Helmkamp et al. (1967), which ensures full preservation of biological activity of the antibody for periods of 2 to 3 months.
It is obvious, that EPAG, unlike RIA, is of little application for mass determinations, as it involves an electrophoretic stage and results take more than a week. We think that EPAG can be used together with RIA when specificity of the latter method needs confirmation.
EPAG is proposed for determining minimal concentrations of AFP when the sample volume and total amount of the antigen are not limiting. There are instances, however, when minimal absolute quantities of AFP have to be determined, such as produced by individual cells or microcolonies. To do this, a modification of the test is required which permits handling microamounts of AFP, and we made an attempt to design such a method based on the EPAG –principle.
Micro-EPAG (Abelev et al. 1974)
In this modification, the examined material is concentrated within a capillary tube, from where the antigen compressed in a 'micro-drop' enters a plate of gel containing specific antiserum. Electrophoresis is stopped immediately the antigen passes into the gel plate. Then the antigen is allowed to diffuse in gel, producing a ring similar to that of the Mancini method (Figures 4 and 5). The area of the ring is proportional to the amount of the antigen in the 'micro-drop'. The sensitivity can be increased by dilution of the antiserum, provided an image of still sufficient contrast is produced on film following treatment with labelled antibody and radioautography.
As little as ~ 50 peg AFP per sample have been detected with micro-EPAG, and this does not seem to be the limit.
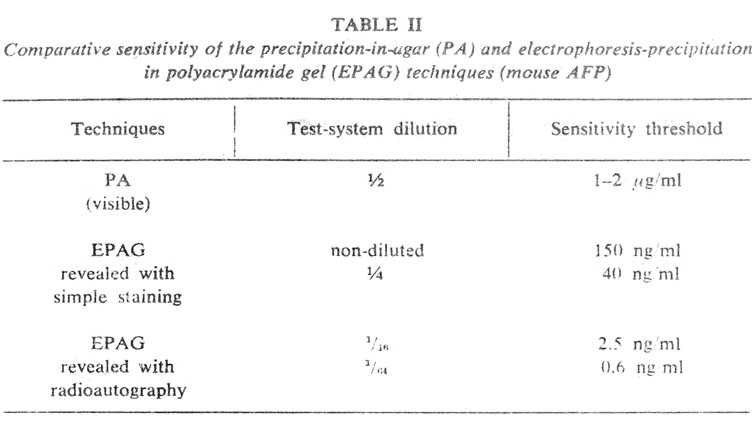
Micro-EPAG is performed in a chamber by two plates with about 0.5-mm clearance between them. Electrophoresis is carried out in two stages. First, the sample, polymerized in 4 per cent PAAG to which two reference substances are added, bromophenol blue (BPB) and hemoglobin (Hb), is introduced into the capillary tubes (0.5 mm inner diameter, 35 mm long) and concentrated using a discontinuous buffer system (Figure 4, I-III). Since rates of migration of the antigen are slightly different in different tubes, while simultaneous entrance into the gel plate is essential for their comparison, the electrophoresis is interrupted each time BPB reaches the end of a capillary tube, the particular tube being removed and transferred to another similar chamber. When all capillary tubes have been transferred to the second chamber, it is filled and polymerised with 5.6 per cent PAAG prepared with the electrode cell buffer containing the necessary dilution of anti-AFP antiserum. Thus, during the second stage of electrophoresis, the buffer is continuous between the electrode cell and the gel plate and discontinuous between the electrode cell and gels in capillary tubes. This provides for uniform and very slow migration of IgG in the plate, while the antigen in the capillary tubes continues rapid movement and enters the free gel simultaneously from all the tubes (Figure 4, IV). The electrophoresis is stopped and the chamber is left for 2 days to achieve free diffusion of the antigen with formation of precipitation rings (Figure 4, V). The latter are revealed after washing by treatment with labelled anti-IgG antibody and radioautography (Figure 5). It seems to be possible to raise the sensitivity of the method by using capillary tubes of a smaller diameter and I125-labelled antibody of greater specific activity.
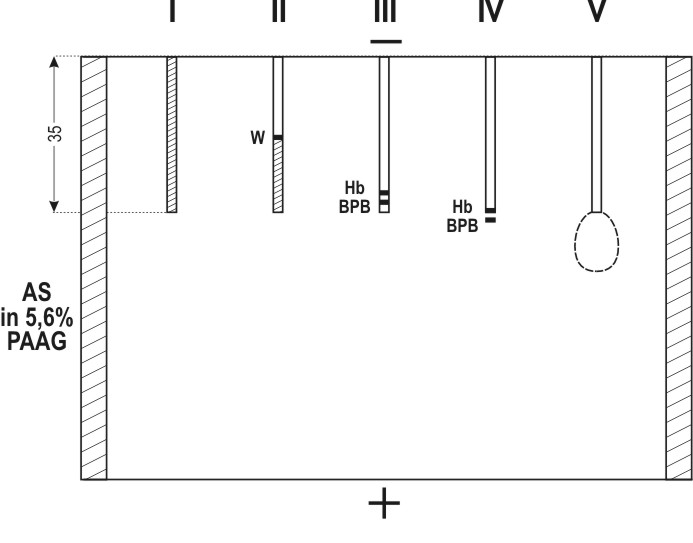
Figure 4. Successive stages of Micro-EPAG technique. I – capillary tube (35 mm long, 0.5 mm inner diameter) filled with 4 per cent PAAG containing the examined sample and reference substances: BPB, bramophenol blue; Hb, hemoglobin. II – concentration of the sample in the capillary; the upper migrating border of the reference substances is visible. III – concentrated sample before exit out of the capillary; concentrated BPB and Hb overlap each other. IV – moment of termination of electrophoresis: BPB has passed into the free gel; Hb is by 1/2 to 2/3 out of the capillary; AFP is under the Hb zone. V – ring of AFP precipitation in the low-porous PAAG containing anti-AFP antiserum. |
Figure 5. A radioautograph obtained by Micro-EPAG technique. The gel contained anti-AFP antiserum diluted 1:1200, the capillaries were filled with PAAG containing newborn mouse serum diluted from 1:640 to 1:10,240, respectively. |
The basic principle of micro-EPAG – concentration in a capillary tube with subsequent entry of antigen into an immunoplate – can also be used for other purposes, such as immunoelectrophoresis of ultramicro-amounts of antigens in PAAG, or combination of such electrophoresis with direct radioautopraphy of protein fractions containing C14 – amino acids.
2. Immunofluorescent localization of AFP in liver and hepatomas
(According to Engelhardt et al. 1969, 1971, 1974)
Attempts to apply immunofluorescence for detecting AFP have been without. success for some time, although it was evident that this localization method should be the principal one in investigations of AFP-producing: cells. AFP, as a well soluble protein, is readily washed out of cells during preparation of slides. Besides, it is present in high concentration in the blood and tissue fluids, from where it can easily penetrate dead cells or cells wit impaired permeability barrier. Hence, the demand for a most adequate fixation procedure and a special control for 'passive uptake' of AFP.
Absolute and 96 per cent alcohol or a mixture of 96 per cent alcohol with acetic acid are poor fixatives for AFP in cryostat sections. The patterns of its distribution appears to be not distinct and most fluorescence is frequently restricted to the contents of vessels. Better results are obtained if cryostat sections are fixed with acetone, as it had been made in the initial study of Gitlin et al. (1967). But the most convenient and adequate technique for immunofluorescent AFP localization seems to be fixation in 96 per cent alcohol with acetic acid followed by embedding in paraffin according to Sainte-Marie (1962) and Hamashima et al. (1964) (Engelhardt et al. 1969). Nishioka et al. (1972, 1973) have arrived to the same conclusion. With this technique, AFP is distinctly localized in AFP-producing tissues both inside and outside cells and fair reproducibility of results is obtained. In addition, an easy way of preparing serial sections is provided as well as the opportunity of repeated examination of the same materials kept in fixed state in paraffin.
To exclude passive uptake of AFP we included detection of endogenous gamma-globulin as a marker of external liquid medium. This protein was chosen because it is not produced by cells of the yolk sac and liver or their tumors and, thus, should not be contained in undamaged cells of the tissues. As far as serum and other biological liquids are concerned, gamma-globulin is present in high concentrations and together with other components may penetrate dying or otherwise damaged cells. We consider AFP localization as physiologically specific, if gamma-globulin is not detected in the same cells by control staining for this protein of neighbouring serial sections. According to our data, such control is essential for proper interpretation of results in work with tumor materials.
To detect AFP, we always used monospecific antibodies isolated from immunosorbents. Fluorescence in sections completely disappeared if the antibodies were neutralized by equivalent amounts of purified AFP, indicating that it was AFP and only AFP that was localized in sections (Figures 6, 7). The proposed method can reveal up to three proteins in serial sections of the same cells, such as, AFP, serum albumin and transferrin (Engelhardt et al. 1972).
|
Figure 6. Immunofluorescent localization of AFP in the neonatal mouse liver. A – liver section of 7-day old mouse incubated with rabbit antibodies to AFP and then with donkey anti-rabbit IgG antibodies labelled with fluorescein isothiocyanate. B – a neighbouring serial section incubated with antibodies to mouse IgG and then with fluorescent anti-rabbit – IgG antibody (x 130). |
|
Figure 7. AFF localization in a transplantable 22a mouse hepatoma. A – detection of AFP as in Figure 6 (A). B – a neighbouring serial section showing specific IgG fluorescent following treatment as in Figure 6 (B). (x 130). |
Association of AFP with teratocarcinomas (TC) of ovary or testicle was simultaneously and independently demonstrated by Masopust et al. (1967. 1968) and by our group (Abelev et al. 1967, Abelev 1968). The protein had been revealed by gel precipitation in about 50 per cent of untreated patients. This finding remained without explanation for a long time. Suggestions were made that TC, being capable of polypotential differentiation, formed analogs of the foetal hepatocyte, that AFP synthesis in TC could be a particular case of the ectopic synthesis in tumors or induced synthesis in the liver (Abelev et al. 1967), or that AFP was a marker of non-differentiated embryonal cells (Kahan & Levine 1972).
Proceeding from the original observations of Gitlin et al. (1967, J972, Gitlin & Boesman J967) that AFP synthesis was effected by the endoderm of the yolk sac, as well as from the fact that structures analogues to the yolk sac were found to be most characteristic of various types of TC (Teilum 1965, 1971, Stevens 1967) we made the suggestion that AFP synthesis in TC was conditioned by development in them of functioning endoderm of the yolk sac (Abelev 1971). Gitlin et al. (1972) also suggested that the endoderm of the yolk sac and of the primary gut, which analogs are present in TC, could determine AFP production by these tumors. This hypothesis has received direct confirmation subsequently and is in good agreement with clinical findings.
First investigation of AFP in human teratocarcinomas by immunofluorescence (IF) was made by Mawas et al. (1969). AFP was detected in the pseudostratified epithelium lining cysts. The type of epithelium was not identified, however. In our laboratory Engelhardt et al. (1973) conducted detailed IF analysis of AFP in a strain of transplantable testicular TC of mice obtained by Dr. Stevens. Apart from non-differentiated cells of embryonal carcinoma, the tumor contained differentiated derivatives of all three germinal layers: nervous tissue, muscles, cartilage, bone, numerous glands lined with different types of epithelium. An important peculiarity of the tumor was its capacity, when grown in the ascite form, to form big and small cysts covered with epithelium resembling the epithelium of the visceral yolk sac. Some of these bodies were very similar to the 6-day-old mouse embryo (Stevens 1967). AFP production by this strain was moderate, about 10 µg/ml in the serum. But localization of AFP were very distinct. AFP was not detectable in non-differentiated cells of embryonal carcinoma, nor in derivatives of the ectoderm or mesoderm. AFP-containing cells were always differentiated and could be characterized in most cases as endoderm derivatives. Most bright luminescence was observed in cells similar to the endoderm of the visceral yolk sac and in the epithelium lining glands and tubules.
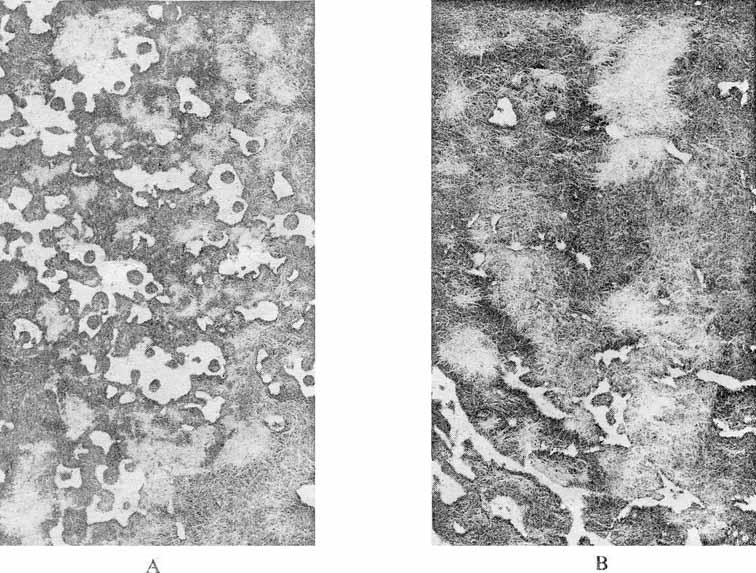
Specific fluorescence, occupied usually an insignificant area of the section and correlated with the blood AFP level of the tumor-bearing host. This correlation clearly emerged from experiments on intraperitoneal inocluation of TC suspensions. In part of mice, this resulted in development of ascites containing freely-floating embryoid bodies and cists. Cells of the outer layer of hese bodies similar to those of the epithelium of the visceral yolk sac contained AFP (Figure 8). The frequency of occurrence of the AFP-containing structures in mice with ascite was higher than in the case of solid tumors and, correspondingly, AFP was found in their blood serum in concentrations up to ten times higher than in serum of mice bearing solid TC.
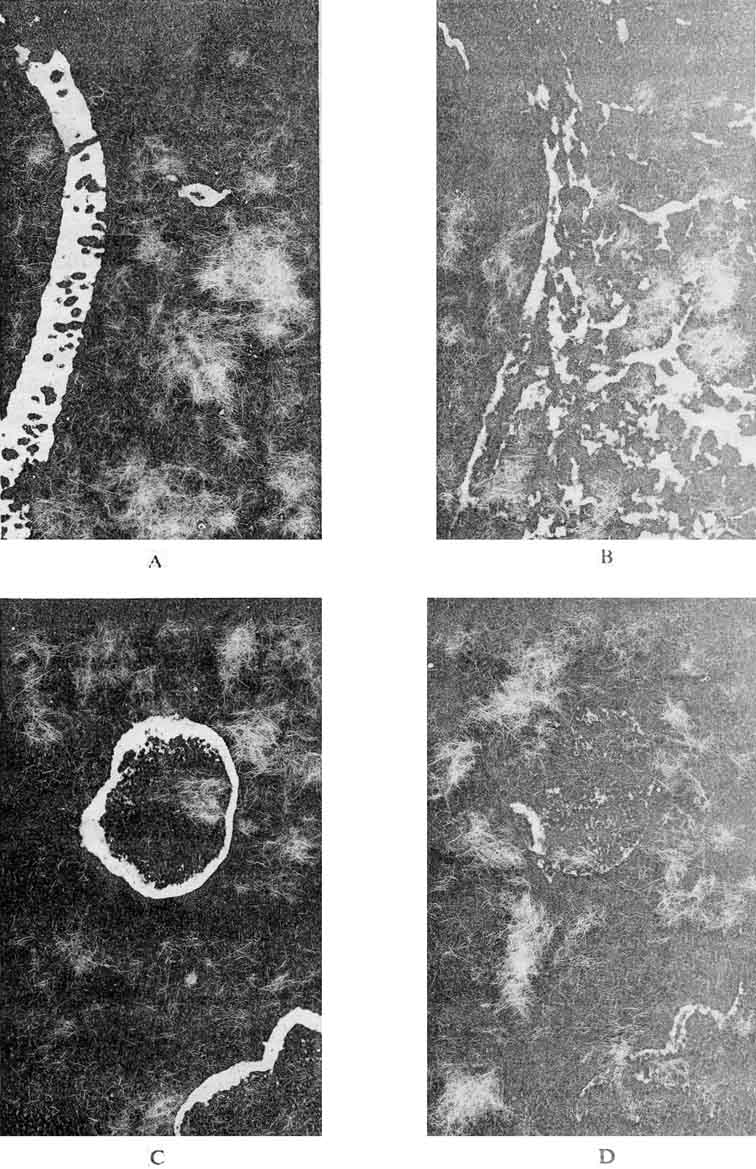
Figure 8. Detection of AFP in sections of a solid teratocarcinoma of the mouse (a and b) and in cysts from the ascite fluid (c). a and c - sections incubated with antibodies for detection AFP; b –section incubated with antibodies for detecting gamma-globulin. (Magnification: a and b x 130, V and d x 36). |
Besides, analysis of cyst contents by immunodiffusion and PAAG electrophoresis showed AFP to be the prevailing component, exceeding albumin and transferrin in concentration. The proportions of the protein components in the fluid inside cysts were much different from those of the surrounding ascite fluid (Figure 9).
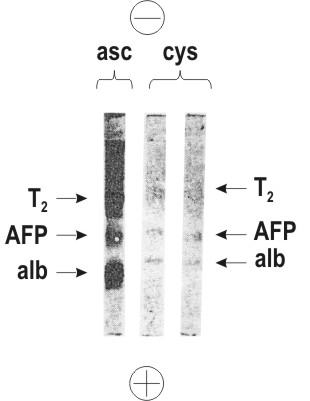
Figure 9. Polyacrylamide gel electrophoresis of the ascite fluid (asc) and of cyst contents (cys) of a mouse teratocarcinoma. Positions of proteins: alb – serum albumin; AFP – alpha-fetoprotein; Tr – transferrin. |
The above findings favour the conclusion that AFP-containing structures in TC represent sites of its synthesis. It is highly probable, in this case, that AFP synthesis is carried out by cells analogous to derivates of early differentation of the endoderm, particularly, by cells of the visceral sheet of the yolk sac in which it is synthesized during normal embryogenesis.
At the same time, TC revealed AFP-containing cells which origin could not yet be identified. Obviously, the above results provide only principal confirmation of the proposed hypothesis and constitute the beginning of systematic studies of AFP in TC, especially in those of man.
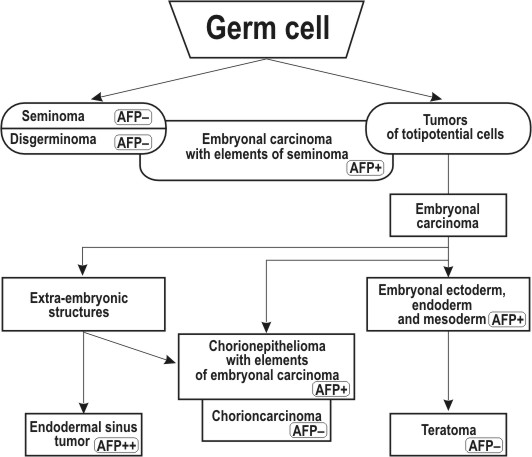
Further analysis of the problem is connected with clinical observations on AFP in various form of germ cell tumors. Histogenesis of germinogenic tumors, their classification based on their natural history, and relations of different types to each other have been most thoroughly studied by Pierce in experimental systems (Pierce & Verney 1961, Pierce 1961, 1967, Kleinsmith & Pierce 1961) and by Teilum (1965, 1971) in clinical material. These tumors, originating from germinal cells, may be tentatively divided into mono- and Polipotential ones. To the first group belong 'pure' seminomas and choriocarcinomas. Seminomas develop from germinal cells, which are strictly determined in the direction of spermatogenesis, and consist mostly of spermatogonia and spermatocyte analogs. In females, disgerminoma corresponds to this form of cancer. Testicular choriocarcinoma is definitely a 'secondary' tumor developing on the basis of polypotential embryonal carcinoma. The stem cell of this tumor most probably corresponds to that determined to differentiate into a trophoblast and capable of independent existence outside embryonal carcinoma on which basis it originated. It is analogous to uterin chorionepithelioma.
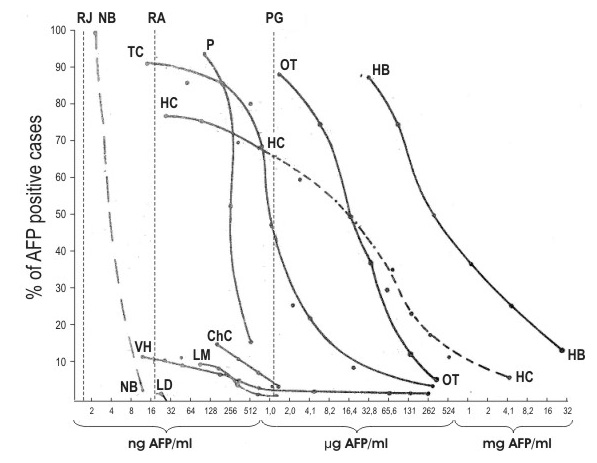
Obviously, no structures corresponding to the visceral yolk sac can develop in tumors of these groups and, accordingly, such tumors should always be 'AFP-negative'. This fully agrees with clinical observations obtained by different investigation groups including ours (Table III, Figure 10). Seminomas and choriocarcinomas are not accompanied by AFP production in any of the sensitivity ranges. However, both seminomas and choriocarcinomas can develop as one of the elements of a polypotential embryonal carcinoma, sometimes the prevailing one. AFP synthesis in such cases could be due to other differentiations within the teratocarcinoma, and AFP production is indeed found in TC with elements of seminoma or choriocarcinoma (Table III, Figure 10).
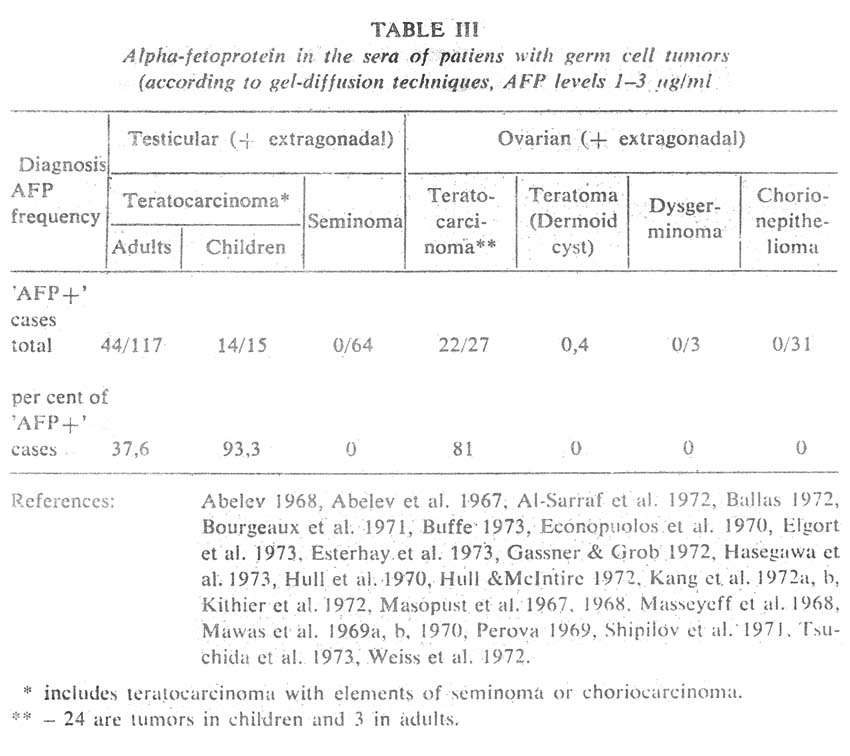
A quite different picture is observed among polypotential germ cell tumors. Testicular TC, which consist of representatives of all the three germinal sheets and possess elements of extraembryonal structures, are accompanied by AFP in 90 per cent of patients (Table III, figure 10). Exeraembryonal elements do not dominate in TC of the testis in adults, which quite agrees with low AFP levels in this disease, compared, for instance, with hepatocellular cancer (Figure 11). The percentage of 'AFP+' cases declines in going from malignant immature TC with elements of embryonal carcinoma to mature teratoma in which 'adult' differentiations prevail (dermoid cysts, Table III). Germinal tumors of the ovary and infantile testicular TG reveal clearly a different picture. A great number of such tumours are embryonal carcinomas with the dominating outgrowth of structures corresponding to extraembryonal elements, that is. primarily, to those of the yolk sac of the embryo (Teilum 1965, 1971, Huntington & Bullock 1970 a, b, Huntington et al. 1963, Pierce et al. 1970).
|
Figure 10. Schematic representation of the expected AFP distrihiition in various forms of germinogenic tumors as related to their histogenesis. AFP– –tumor form not producing AFP; AFP+ – moderate AFP levels; AFP++ – highly positive form both in AFP level and frequency of AFP detection. |
And it is precisely embryonal carcinomas of the ovary and infantile testicular TC that stand out for the highest levels of AFP production and high percentage of 'seropositive' patients (Table III, Figure 11). Obviously, this tumor group is most interesting for direct immunofluorescent studies, especially the so-called poly vesicular tumors of the ovary (Teilum 1971).
Thus, consideration of germinogenfc tumors shows that the resumed AFP synthesis in them is caused not by profound 'dedifferentiation' but rather by their ability to differentiate progressively into functioning embryo-specific structures corresponding, most probably, to the endoderm of the yolk sac which fulfils this function during normal embryogenesis. If this suggestion is confirmed conclusively clinical immunodiagnosis of germ cell tumors will get a quite rational basis.
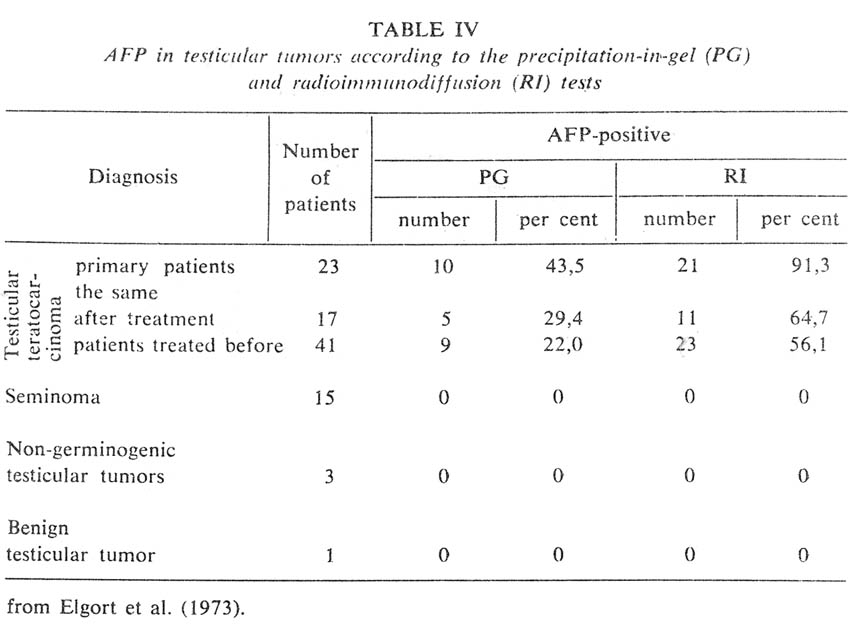
Concluding this section, I would like to consider briefly the clinical aspects of immunodiagnosis of TC. This diagnosis proved its value both in cases of children and adult germinal tumors. AFP levels in children tumors are very high, as a rule, and quite detectable by the ordinary methods of immunodiffusion. However, these methods reveal AFP in less than 50 per cent of testicular TC cases of adults (Elgort et al. 1973, Table III). Application of the highly sensitive RI test nearly doubles the percentage of revealed tumors (Table IV). This greatly improved effectiveness of diagnosis is practically not accompained by any decrease in specificity, because liver diseases also 'captured' in this case (hepatitis and metastatic liver cancer) (Figure 11) do not usually constitute the object of differential diagnosis from tumors of the testis or ovary and can be excluded on the grounds of clinical signs.
|
Figure 11. Percentages of AFP-positive patients revealed by the AFP test as dependent on serum AFP levels. Vertical broken lines represent the sensitivity thresholds of the agar-gel precipitation (PG), radioimmunodiffusion (RA), and radioimmunoassay (RI) techniques. NB –normal background according to Rudslahti & Seppälä (1972 a, b); LD –noncancerous liver diseases (129 cases); VH –viral hepatitis (259 cases); LM –metastatic liver cancer (76 cases); ChC - cholangiocellular carcinoma (26 cases); TC - testicular carcinoma in adults (23 cases); HC –hepatocellular carcinoma (43 cases); Hb –child liver cancer (8 cases); OT –ovarian teratocarcinoma (16 cases); P –pregnancy (47 cases). The dotted part of HC curve represents suggested distribution based on comparatively insufficient quantitative data. |
At the same time, presence of AFP enables one to distinguish correctly TC from 'pure' forms of seminoma or chorionepithelioma or from malignancies of the testicular stroma. If, however, the elements of seminoma or choriocarcinoma prevail only in a polymorphous TC, presence of AFP is an important indication of that. In adults practically absolute specificity of the diagnosis is apparent, even with the highly sensitive RI test of AFP detection. Precise differential diagnosis between seminomas, choriocarcinomas, and TC is clinically important because different chemotherapy is required with these forms of cancer.
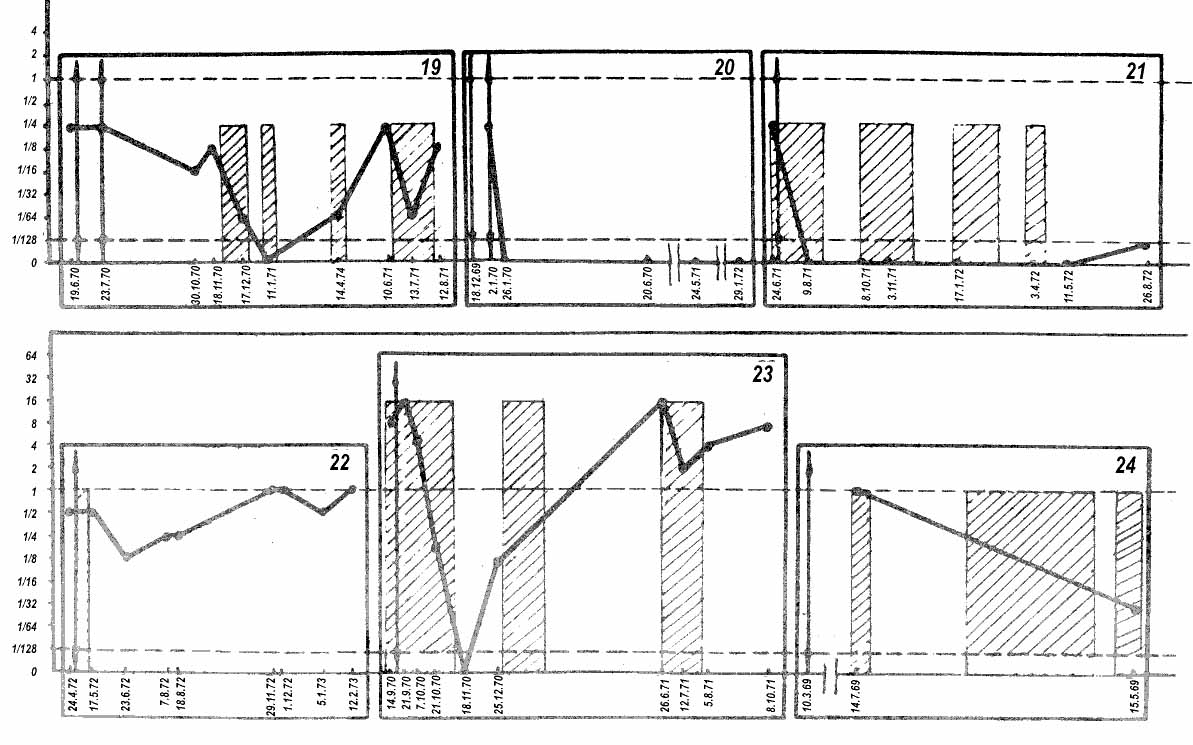
Another significant advantage of Rl test with TC is the possibility to evaluate effectiveness of treatment and to make a prognosis. AFP levels in TC cases are usually not high even if they are detectable by standard immunodiffusion tests. Upon surgery or chemotherapy, AFP 'escapes' into the subthreshold zone and becomes detectable again only after vigorous progress of the disease. The entire dynamics of AFP changes usually takes place in the 'sub-treshold' area which is quite accessible to RI test, there being good correlation between the AFP level and rates of development of the tumor or its metastases (Elgort et ah 1973). Figure 12 shows several curves which characterize the correlation between AFP level and progress of the disease. Two reservations should be born in mind, however, in such comparison. First, although there is a principal correlation between serum AFP level and size of the tumor in the particular patient, the ratio of AFP to tumor size is quite different in individual patients and, probably, in tumor and metastases. Secondly, a relapse or metastasis of an originally AFP-positive tumor can be with "late" AFP production or without it in a proportion of cases (about 10 – 15 per cent of patients) (Elgort et al. 1973, Braunstein et al. 1973).
|
Figure 12. Dynamics of AFP in patients with testicular teratocarcinomas. Abscissa: dates of testing. Ordinata: AFP levels presented taking the sensitivity of the visible test-system (1-2 µg/ml) as unity. Arrows indicate dates of surgey. Hatched rectangulars represent periods of chemotherapy. Horizontal broken lines indicate the sensitivity thresholds of gel precipitation (upper line) and radioimmunodiffusion (lower line) methods. |
Thus, investigation of quantitative aspects of AFP in the clinic has permitted, on the one hand, to bring patterns observed in germ cell Surreal basis and, on the other hand,, to understand clearly the relationship between sensitivity and specificity of the immnuodiagnostic test in teratoearcinomas and its usefulness in evaluation of the effectiveness of treatment of tumors.
Whereas with germ cell tumors resumption of AFP synthesis is getting a plausible explanation, the position seems to be much more complicated in case of hepatomas. First of all, it remains completely obscure which hepatocyte form is responsible for AFP synthesis. We definitely know, only, that it is exactly foetal or early postnatal hepatocytes, as well as their malignant analog, that are involved (for review see: Abelev 1968, 1971). But, to be found is what distinguishes the AFP-producing hepatocyte from a non-producing one, whether in the liver or hepatomas, and why AFP production may differ a thousand-fold in the same type of liver cancer. Whether AFP synthesis is connected with a special population of foetal hepatocytes, the nearest derivatives of the gut endoderm, or with an early phase of differentiation of every hepatocyte, and whether the synthesis requires an external 'inducer', – all these issues constitute a non-elucidated problem. Without its solution it is hardly possible to get a closer understanding of the nature of the phenomenon.
Important material on the cellular basis of AFP production can be obtained by comparing data on AFP dynamics during physiological and pre-cancerous regeneration of the liver.
AFP synthesis is resumed in adult animals, and possible in man, too, during active liver regeneration caused by hepatectomy, CCL4 poisoning, or viral hepatitis. The resumption is for short periods, and AFP levels vary significantly depending on the species, age, and even genotype of the animals.
Thus, mice exhibit most expressed induction of AFP synthesis. Hepatectomy (Abelev et al. 1963), inhalation of GCL( (Bakirov 1968), or its injection (Pihko & Ruoslahti 1973) produce increased AFP levels by the second day, which reach maximum on the third or fourth day and gradually decrease to the background level in subsequent 8 to 10 days. The AFP levels are high: they are determinable by the immunodiffusion test in blood serum samples diluted 1:2 to 1:16, which means the range of 5 to 50 µg/ml. Strong dependence on the animal strain has been demonstrated (Abelev 1968, 1971, Pihko & Ruoslahti 1973). An example of contrast strains are C3H (high producers} and C57BL (low producers) mice in which the serum AFP level during regeneration differs by one order of magnitude, the difference being levelled in young animals (up to 2 months).
In rats, hepatectomy results in 100 per cent AFP induction with the dynamics closely resembling that of mice but with much lower serum AFP levels: about 0.5 µg/ml (Perova et al. 1971). In young rats – fifth or sixth week of postnatal development – a steep wave of AFP synthesis is produced, just like in mice, by hepatectomy (Perova & Abelev 1967) or CCL4 (de Nechaud & Uriel 1971).
Hepatectomy in humans is sometimes accompained by AFP synthesis (Smith 1971, Matray et al. 1972, Ruoslahti & Seppala 1972) but observations are episodical and contradictory (Alpert 1972). There are more numerous data on viral hepatitis which is regularly associated with liver necrosis and subsequent regeneration of the liver. Here, the increase in AFP is observed in no more than 15 per cent of patients, most of the positive cases possessing levels less than 0.5 µg/ml with production lasting for 2 to 4 weeks (Figure 11) (Abelev et al. 1971, Ruoslahti & Seppälä 1972, Nishi & Hirai 1973).
The cellular aspects of AFP synthesis during regeneration of the liver have not been studied, but certain preliminary considerations are possible on the basis of purely phenomenological analysis.
AFP production by regenerating liver does not seem to be connected with the main rehabilitation process. It is most probably determined by some 'minor' component of the process varying with age, species and even genotype of animals. There seem to be cases of regeneration which proceed without participation of the supposed 'minor' component. These considerations are supported by the obvious lack of correlation between the occurence and degree of the regeneration process, on the one hand, and the intensity of AFP synthesis, on the other hand.
Another point, substantial for further discussion, lies in the fact that differend species and even genotypes of animals are distinguishable by the degree of AFP 'induction' during regeneration of the liver; in other words 'inducibility' of AFP in adult animals seems to be under genetic control.
A quite different picture is observed during liver regeneration which takes place in the acute phase of chemical carcinogenesis. Watabe (1971) and Kroes et al. (1972) have shown that AFP synthesis during this stage of action of hepatocarcinogenes takes the form of a steep wave fading to the time of appearance of hepatomas and rising again with development of tumors. Both the frequency and level of AFP synthesis are in positive correlation with the carcinogene 'power' and dose. The synthesis is not induced, as a rule, by non-carcerogenic analogs of the agents (Kroes et al. 1973, de Nechaud & Uriel 1973, Kitagawa et al. 1972, 1973, Kitagawa & Sugano 1973, Hirai et al. 1973).
Thus, the early AFP wave in chemical carcinogenesis is most probably a marker of a specific phase in the process. It is significant that AFP levels in rats during that wave are 50 to 100 times higher than during physiological regeneration.
It may constitute a substantial and perhaps decisive distinction that the 'minor' component of physiological regeneration becomes a leading one during regeneration preceeding cancerogenesis. Comparison of the two processes shows that, in the former, rehabilitation of the liver proceeds mainly by proliferation of mature preexisting hepatocytes (Leduc, 1964), whereas during poisoning of the liver with a carcinpgene and regeneration in its presence, new hepatocyte formation takes place from the precursor cells with complete renewal of the hepatocyte population (Price et al. 1952, Farber 1956, Kitagawa et al. 1972, 1973).
Detailed analysis of cellular population dynamics in the liver has shown that maximal AFP production correlates with mass transition of the so-called oval cells into small hepatocytes (Iwasaki et al. 1972). It is a well-substantiated suggestion that the oval cell may be the precursor of the hepatocyte which first transforms to the small hepatocyte and the latter differentiates into the big, mainly polyploid hepatocyte characteristic of the adult liver (Wilson & Leduc 1958, Leduc 1964, Ianoka 1967, Iwasaki et al. 1972). Onoe et al (1973) suggested that AFP is produced by a transitional form from the Oval cell to the small hepatocyte, the latter maintaining AFP synthesis for a short period.
It is not possible to decide from the available evidence whether AFP production denotes a stage of differentiation taking place in every hepatocyte or just a 'blind' branch in differentiation resulting in a separate short-living population of embryo-specific small hepatocytes. The evidence permits both possibilities.
Another point substantial here is – that AFP production is associated with activation of the precursor cells those very cells which are most probable 'targets' in tumor transformation resulting in tumor stem cells.
It would be superfluous and premature to specify right now at which differentiation stage the precursor cell becomes the target cell for transformation. At present, to specify also whether precursor cells themselves possess AFP or their close progeny would be equally premature. Suffice is to state that a necessary relationship exists.
There are some direct and indirect indications that only oval cells or transitional forms produce AFP (Kitagawa et al. 1972, Onoe 1973, Onoe et al. 1973, Uriel et al. 1973). Oh the other hand, IF studies made in our laboratory on AFP localization in the fetal and postnatal liver have clearly shown AFP presence in typical hepatocytes (Engelhardt et al. 1969, 1971, 1974, Shipova et al. 1974) (Figure 6). Besides, massive proliferation of oval cells induced by α-naphthyl-isothiocyanate with the following differentiation of these cells into bile ductules (Lopez & Mizzanti 1955, Steiner & Carrathers 1963) is not accompanied by AFP production (Kroes et al. 1973).
The suggestion of AFP as a marker of target cells which emerge from the precursors in the process of new hepatocyte formation makes it possible to relate many apparently unconnected groups of observations, as well as to derive some concrete consequences. It explains, in the first place, the specific relationship between the carcinogenic activity of a substance and its capacity to induce AFP in the precancerous period.* One may think that the initial carcinogene action is as follows: being selectively 'toxic for mature differentiated cells and causing their degeneration, the carcinogen specifically stimulates proliferation of precursor cells and thus increases the target-cell population for tumor transformation; The transformation proper may be due to the action of an activated virus, or the carcinogene itself, or spontaneous, but a critical number of target cells is certain to be created first by the carcinogene. Kambial elements are negligible in the adult animal liver and the effect of hepatocarcinogene may consist, in the first place, in selective stimulation of these elements. And this is signalled by the first wave of AFP synthesis.
From these positions, physiological regeneration of the liver may stimulate proliferation of precursor cells, but this 'miner' component is only accompanying rehabilitation of the liver which proceeds, principally, by division of mature hepatocytes.
Karyological peculiarities of hepatocellular carcinomas, also become understandable from the above positions. Experimental hepatomas are known to consist, as a rule, of diploid or near-diploid cells in rats and mice (Nowell et al. 1967, Guelstein 1966). This is at obvious variance with the absolute preponderance of polyploid cells in the adult livers but quite agrees with the above supposition about the hepatocyte precursor-cells or their nearest progeny serving as target cells in tumor transformation.
The hypothesis on correlation of AFP with activation of the precursors explains well the 'unilateral' dependence of AFP production on proliferating processes in the liver. It is evident that the necessary condition should be cellular proliferation leading to new hepatocyte formation. And, indeed, AFP is being produced exclusively under conditions involving active proliferation of liver cells: the foetal, neonatal, and regenerating liver, the state of pre –cancerous hyperplasia, hepatomas. On the other hand, it is known that active liver cell proliferation in rats during the late postnatal period and regeneration occurs on the background of a sharply declining AFP level or with a very low intensity of the synthesis (Abelev et al. 1967 b, Perova et al. 1967, 1971, de Nechaud & Uriel 1971).
| * Possible exception is diethylnitroseamin in rats (Kitagawa et al. 1973, Kroes et al. 1973). |
As already mentioned, here one can think about insufficient involvement of the new hepatocyte formation process in the growth or regeneration of the liver.
A good explanation is provided, within the above hypothesis, to greatly different frequencies of spontaneous hepatomas in mice and rats. Mice possess not noly considerably higher AFP background than rats or humans (Abelev 1971, Pihko & Ruoslahti 1973), but much greater inducibility of AFP, too. The occurrence of spontaneous cancerogenesis in mice seems to correlate not so much with the higher background level of AFP, as proposed by Pihko & Ruoslahti (1973), but rather with the greater inducibility of the protein. The high-cancerous C3H strain does not differ from the low-cancerous C57BL strain in AFP background, high in both cases, but does so by the inducibility of AFP subsequent to hepatectomy or CCL4 treatment. If AFP level indeed reflects the 'volume' of target-cell population, C3H mice should not possess a greater initial pool of such cells, but more ready ways of their 'mobilization'. Even such particular features as the carcinogenicity of CCL4 for mice, but not for rats, appear quite consistent with the observed differences in AFP inducibility.
The experimental evidence mentioned in connection with the precursor-cell activation hypothesis permits a new outlook and explains partially the significance of finding AFP in non-cancerous diseases of man. It seems that liver regeneration in viral hepatitis and cirrhosis may involve the precursor-cells in a quite different degree. In regeneration accompanied by AFP synthesis, one can expect an activation of the precursors and such regeneration can be considered as close to the precancerous one.
Elevated serum AFP levels in healthy individuals from "high-risk" regions (Purves et al. 1973 a, b), as well as in various liver and non-liver pathologies (Elgort et al. 1972), may be the evidence of an enlarged initial pool of the precursor-cells or of an easiness of their activation. This could be one of the basic reasons for high frequency of liver cancer among certain human populations.
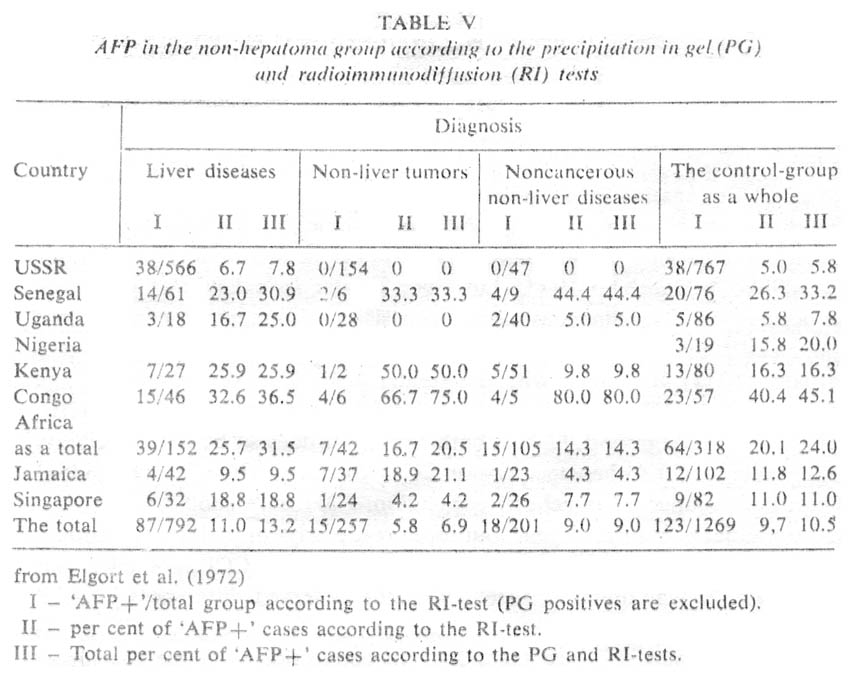
In the case of high-risk groups, it is not simply the elevated AFP background that should be mentioned, but rather the easiness of AFP induction. Thus, Purves et al. (1973 a, b) have observed a bimodal type of AFP distribution among Mozambique Bantu who had arrived directly from high-risk areas. One maximum corresponded to normal AFP level, while the other one corresponded to elevated levels. After a year on a common diet, the second maximum disappeared. It is interesting that the frequency of elevated AFP levels among patients suffering from various diseases, excluding liver cancer, appeared to the greatly increased, which has never been observed among patients in Europe or in the USA. According to our data obtained for groups of patients from different African countries, also, there was up to 45 per cent positive cases in non-hepatomic groups with most serum AFP levels in the range of 0.1-0.5 µg/ml. There was no correlation between such cases and liver diseases, which has been directly opposite, to results obtained for USSR patients, in whom AFP in the range of 0.1-0.5 µg/ml was encountered only with viral hepatitis and metastatic liver cancer (Table V, Figure 11). It would be most important to have comparative data on AFP in viral hepatitis for regions with high and low frequency of hepatocellular cancer. Such data would permit direct characterization of the 'inducibility' of AFP as dependent on predisposition to primary liver cancer in the particular population.
The hypothesis about causal relationship between the precursor cells and AFP permits a number of specific predictions to be made that are accessible to experimental testing:
(a) It is to be expected for C3H and C57BL strains of mice, which contrast in the 'inducibility' of AFP, that their oval cells should display different degrees of proliferation in response to hepatectomy or CCL4 treatment. If this appeared to be the case, inducibility of AFP should be inherited in F1 (C3H x C57BL) and in F2 progeny coupled with inducibility of the oval cells and the frequency of spontaneous hepatomas. Such results, if available, could be regarded as an absolute proof of the considered hypothesis.
(b) It is to be expected that repeated hepatectomies in C3H mice, but not in C57BL, should stimulate spontaneous cancerogenesis in this strain.
(c) In the rat strain for which CCL4 is a hepatocarcinogene (Reuber & Glover 1970), the agent should induce AFP.
(d) Inducibility of AFP subsequent to hepatotoxic treatments should be greater in populations of high risk for primary liver cancer. Patients whose elevated AFP levels are associated with hepatitis or chirrhosis might constitute a high-risk group compared with corresponding 'AFP-negative' patients.
Thus, the field of physiological and pre-cancerous regeneration seems to be most promising at present from the point of view of the opportunities to identify liver cells responsible for AFP production and to determine their relationships with the tumor precursor-cells. Combined application of IF, histochemical, and genetic analysis should help in solving these problems and to gain further insight into the processes of chemical and spontaneous cancerogenesis of the liver.
Although this field has been investigated fairly well by now, it does not appear possible yet to provide explanation for accumulated facts from more or less strict positions. On rather, they can be explained equally readily from mutually-excluding points of view. First of all, we should like to formulate what has to be explained. This may be done by the following listing of groups of facts:
(a) Extremely great (a thousand-fold) difference in AFP levels between individual cases of hepatocellular cancer ranging from 20 ng/ml to 15 mg/ml (See: Masseyeff et al. 1968, Abelev 1971, Ruoslahti et al. 1972. Ruoslahti & Seppälä 1972 b). Existence of hepatocellular carcinomas not producing AFP. In countries with low incidence of hepatocellular cancer such tumors constitute up to 20 per cent of histologically proved cases (Ruoslahti & Seppälä 1972 a, b, Elgort et al. 1972) (Figure 11). Simultaneous existence, on this background, of a clearcut group of embryonal liver tumors, the hepatoblastomas (Ishak & Glunz 1967, Kasai & Watanabc 1970). which stand out for both frequency of seropositive cases and AFP levels (Buffc 1973. Hasegawa et al. 1973, Hull & Mclntire 1972, Mawas et al. 1969, 1970) (Figure 11).
(b) Positive correlation between the frequency of "AFP+' cases of hepatocellular cancer and the incidence of the disease in the particular geographical area (See Abelev 1971, Masseyeff 1972). This regularity is maintained at all sensitivity ranges of AFP determination.
(c) AFP production .by only apart of tumor cells (Goussev et al� 1971, Nishioka et al. 1972, 1973, Engelhardt et al 1974), and the correlation of the production level with the number af 'AFP+' cells in the tumor (Engelhardt et al. 1974).
(d) Existence of some slight correlation between the degree of tumor differentiation and the AFP production by the tumor. AFP is less frequent in highly differentiated tumors of man than in moderately differentiated tumors, but becomes less frequent again in low-differentiated tumors (Purves et al. 1970, Sakurai & Miyaji 1973). The correlation scenes to be more expressed in experimental liver tumors of mice (Abelev 1968, 1971) and rats (Kitagawa & al. 1972, 1973).
It is obvious that in order to understand what is taking place in hepatomas, the relationship of AFP-producing cells to the tumor stem cells should be determined. One can visualize three most general possibilities in this respect, all being principally compatible with the above mentioned facts:
1. The tumor stem cell may be considered as originating from the hepatocyte, at a certain stage of its differentiation, and maintaining that stage in the tumor. If the stage is 'AFP-positive', the tumor continues to produce AFP.
2. The tumor stem cell is derived from a determinated but not differentiated precursor-cell of the hepatocyte. It is capable of differentiation into the hepatocyte. AFP being produced by 'transitory' forms. Differences in AFP level reflect the pro portion of 'transitory' forms in the tumor.
3. The tumor stem cell originates from a determinated but not differentiated precursor-cell of the hepatocyte, capable of developing in two alternative directions: towards the fetal type hepatycote, which produces AFP, and towards the mature hepatocyte synthesizing 'adult plasma' proteins. The proportions of 'branches' are determined by stem cell properties and environmental conditions. The level of AFP production reflects the proportion of the fetal hepatocyte analogs in the tumor.
In our studies, we confine ourselves to the latter hypothesis, considering that it most naturally explains the groups of facts mentioned at the beginning of this section (Abelev 1968, 1971, 1973). A real problem, however, is to choose experimental approaches to test the above alternatives. Two ways may be used for this purpose, in our opinion. The first approach, a direct one, is to isolate individual cells from an ascitic hepatoma and to determine the synthesis of AFP and other serum proteins by these cells, as well as their ability to form clones or micro-colonies in vitro or to develop tumor in vivo.
We hope that this approach may be realized by application of the micro-EPAG test. Of course, it would also be very interesting to examine cell clones at different stages of their development by IF analysis.
The other approach is an immunological one. Adult animals are tolerant to AFP because of the high level of the protein during the period of immunological maturation and the presence of its background level in the adult organism. The tolerance to AFP can be readily terminated by immunization of animals (rats, rabbits, horses, but not mice) with cross-reacting AFP belonging to a related animal species, for example, by immunization of rats with human or mouse AFP (Nishi et al. 1972, Goussev & Jazova 1974). When the tolerance is overcome, rats respond by active antibody formation to their own AFP. One can expect that the active immunity against AFP would supress the growth of AFP-producing cells, as it occurs in case of plasmocytomas transplanted to mice immune to the idiotypic determinants of IgG produced by the particular plasmocytoma. If AFP is in some way connected with the stem cells of the hepatoma, suppression of tumor growth can be expected; if it is not connected, one can expect selective inhibition of the differentiation process towards the AFP-producing cells.
Of similar interest is to investigate possible effects of immunity against AFP on chemical carcinogenesis in the liver: will the target-cells be suppressed, or the character of developing tumors be changed? Such experiments are being made at present in various laboratories of the world. It is obvious that the results of these experiments will also determine the prospects of immunotherapy of hepatomas based on termination of tolerance to AFP.
In the present section we do not consider the clinical aspects of liver cancer immunodiagnostics. This issue has been exhaustively discussed in a number of recent reviews and papers (Tatarinov 1970, Abelev 1971, Masseyeff 1972, Hirai & Miyaji 1973, Anderson et al. 1972, McIntire et al. 1972).
Our data are summarized graphically iri Figure 11 and in Table V. They are typical of countries with low frequency for primary liver cancer and provide a fairly complete picture on the diagnostic specificity of AFP test as dependent on its sensitivity.
The clinical application of AFP test to the diagnostics of liver cancer has gone far ahead of the study of its biological aspects. One may hope that basic researches in the field of AFP would stimulate new approaches to the mechanisms of hepatocarcinogenesis and probably to immunotherapy of this type of malignancy.
The valuable help of Drs. D. Elgort, S. Perova and N. Engelhardt in the preparation of this manuscript is greatly acknowledged.
My sincere thanks to Dr. L. Mekhedov for the English translation of the paper and to Miss. M. Berdichevskaya for the technical assistance in its preparation. The experimental studies referred here were partly supported by WHO Immunology Unit.

E. Garfield. The Russians Are Coming! The Top 50 Soviet Papers Most Cited in the 1973-1988