| Home page (in Russian) | Texts in English | Guestbook |
Int. J. Cancer: 17, 798-805 (1976)
E. S. Ievleva, N. V. Engelhardt and G. I. Abelev
Laboratory of Tumor Immunochemistry,
N. F. Gamaleya Institute of Epidemiology and Microbiology,
Moscow, USSR
Received: December 3, 1975, and in revised form March 30, 1976.
A new antigen has been revealed by means of antisera against Rauscher virus in mice with Rauscher virus-induced leukemia. This antigen appears to be different from both Rauscher type-specific antigen and MULV-gs-I (p-30), as shown by studies of electrophoretic mobility and immunochemical specificity. Except in leukemic mice it was also found in low levels in both serum and spleen extracts of healthy mice of a number of strains. Furthermore, this antigen was regularly demonstrated by immunofluorescence on the surface of erythroblasts, but not on the surface of erythrocytes, lymphocytes, polymorphonuclear cells and thymocytes, and was shown to be different from fetal hemoglobin. Therefore, it is referred to as antigen of erythroblasts (Ag-Eb), which seems to represent a surface marker for a certain differentiation stage of erythroid cells. |
Several antigens are known to be specific for cell surface of murine lymphoid cells – MSLA (mouse-specific lymphocyte antigen) (Shigeno et al., 1968); and four genetically determined alloantigens, Ly-1, Ly-2, Ly-3 and Ly-4 (Schlessinger, 1972; Boyse et al., 1971, 1968; Snell et al., 1973) are present on lymphocytes and thymocytes. Besides these antigens there are antigens peculiar to thymocytes only: TL-antigen (Boyse et al., 1964) and Thy-1 antigen (Reif and Allen, 1964). Plasma cells also have an antigen which is retained in plasmocytomas – PC-1 (Takahashi et al., 1970). All these antigens represent the antigens of the hemopoietic cell differentiation and could be used as specific markers for surface structures of a certain cellular type.
The present paper considers immunochemical properties of one more antigen, revealed in mice with Rauscher virus-induced leukemia. This antigen proved to be specific for mouse erythroblasts and was designated Ag-Eb (Ievleva et al., 1974).
Mice
Mice of the inbred strains C57B1/He, C57Bl/10Sn, BALB/c, C3H/Sn, CC57Br, CC57/W were obtained from the inbred animals laboratory of this Institute, AKR and C57B1/6 from the nursery of AMS of the USSR, and Fl (C57Bl x CBA) hybrids from the Central Institute of Blood Transfusion, Moscow.
Tumors
Rauscher leukemia was maintained in BALB/c mice by intraperitoneal injections of a leukemic spleen cell suspension (Abelev et al., 1968).
Mice with Friend, Moloney, Stepina-Zilber and Mazurenko virus-induced leukemias were kindly provided by Dr. V. N. Stepina (Institute of Experimental and Clinical Oncology, Moscow).
Mice with 9, 10-dimethyl-1,2 benzanthracene-induced leukemias (BL-15 and KL-2) were obtained through the courtesy of Dr. Z. A. Postnikova of this Institute.
Murine Rous-virus-induced tumors were kindly provided by Dr. I. N. Kryukova, polyoma-virus-induced tumors by Dr. I. S. Irlin, and methyl-cholanthrene-induced sarcomas by Drs. O. M. Lejneva and B. D. Brondz of this Institute.
Tissue extracts
Extracts were prepared by homogenization of the tissues in three volumes of Ringer's solution.
To prepare erythrocyte lysates, blood was collected from six to eight murine fetuses after about 17-19 days of gestation. The washed erythrocytes were counted in a hemocytometer and lysed with distilled water. Two samples contained 3.5 x 108 erythrocytes in 0.2 ml, and two other samples contained 3.5 x 108 erythrocytes in 0.4 ml. The lysates were centrifuged for 20 min at 5,000 x g and the supernates were reduced to a quarter of their original volume by lyphogel (Gelman, England).
Colonies of hemopoietic tissue
Colonies were obtained by the method of Till and McCulloch (1961). F1 (C57Bl x CBA) mice were irradiated (radiation dose – 850 rad, source of radiation – 137Cs, radiation rate – 26 rad/min), and then received intravenously syngeneic bone-marrow cells (3 x 104 per mouse). Eight to ten days later the mice were killed and their spleens were removed. The spleens exhibited fairly visible discrete colonies, which were morphologically characterized either after staining the imprints according to Romanovsky-Gimza or through examination in a hemocytometer of the suspension of dispersed cells, stained with 1% trypan blue. It was easy to distinguish granulocytic cells from erythroid cells by the shape of their nuclei.
Rauscher virus
Virus was purified from the blood plasma of mice with established leukemia by sucrose density gradient centrifugation. Zones with density 1.14 and 1.16 were isolated (Ievleva et al., 1969).
Commercial preparation of Rauscher virus
Virus (Electro-Nucleonics Laboratories, Inc., NCI, Bethesda, USA) was produced in the fibroblast-like JLS-V9 cell line and purified by JLS-V9-RLV double density gradient zonal centrifugation. Virus count was 2.9 x 1011 particles per ml. MuLV-gs-1 titer in non-destroyed virus according to complement fixation test is 1:256, in lysed virus 1:1024.
Immunization
Rabbits were injected into the popliteal lymph nodes with a purified preparation of Rauscher virus, emulsified with complete Freund's adjuvant (1 +4). The immunizing dose contained 30,�g of protein, estimated by the Lowry method (Lowry et al., 1951). One month later the animals were injected intravenously and intramuscularly with the same dose of material. Bleeding was repeated from the ear vein several times, starting from the 7th day after the second injection (levleva et al., 1969).
The test system for MuLV-gs-1 (p30)
The system was composed of monospecific rabbit antiserum against MuL V-gs-1 (p30), kindly provided by Dr. A. I. Goussev, and equivalent dilution of spleen extract from a mouse with Moloney virus-induced leukemia.
The precipitating test system for type-specific antigen of Rauscher and Freund viruses
The test system was provided by Dr. O. M. Lejneva. It was composed of rabbit antiserum against Rauscher virus (undisrupted and equivalent dilution of spleen extract from a mouse with Rauscher virus-induced leukemia (Lejneva, 1974).
Double diffusion
The test was performed in microgels according to Goussev and Tsvetkov (1961).
Highly sensitive radioimmunodiffusion method
The method was employed to reveal invisible precipitin lines (Abelev and Elgort, 1970; Abelev et al., 1974).
Analytical Immunoelectrophoresis
Immunoelectrophoresis was performed in 1% agarose on 25 mM barbiturate buffer, pH 8.6.
Partial purification of the antigen
Purification was achieved with preparative electrophoresis in agarose (Zilber and Abelev, 1966; Abelev et al., 1968). Spleens from mice with Rauscher virus-induced leukemia were homogenized in Ultra-Turrax in Ringer solution 1 + 3. The extract was centrifuged for 30 min at 20,000 x g, concentrated to 1/6 of the original volume by polyethyleneglycol (mol. wt 40.000) and dyalized against barbiturate buffer, pH 8.6. Fractions, obtained after preparative electrophoresis, were concentrated to 1/5-1/6 of their original volume, using Sephadex g-50 medium, or polyethyleneglycol. Ag-Eb and MuLV-gs-1 were revealed in these fractions in double diffusion in gel with the corresponding test-systems.
Isolation of antibodies to Ag-Eb
Antibodies against Ag-Eb were isolated by partial dissociation of the specific precipitate in acid medium (Abelev and Avenirova, 1960). In separate experiments we estimated the amount of spleen extract from mice with Rauscher leukemia sufficient to neutralize 0.1 ml of monospecific antiserum to Ag-Eb. Spleen extract was clarified by centrifugation for 20 min at 9,000 x g and monospecific antiserum to Ag-Eb for 30 min at 13,000 x g. These components were mixed in the calculated ratio, incubated for 40 min at 37° C and left for 36-48 h at +4° C for complete formation of the precipitate. The precipitate was thoroughly washed three times by resuspension in pre-cooled neutral saline, followed by centrifugation for 10 min at 350 x g. It was then suspended in 0.1 m phosphate-citrate buffer pH 3-3.2 and left at room temperature for 40-60 min. The buffer volume usually equalled 1/10 of the volume of the antiserum employed to obtain the precipitate. The remainder of the precipitate was separated by centrifugation and the supernatant with eluted antibodies was carefully collected, neutralized by addition of dry NaHC03 till pH 7.2-7.4 and tested in double diffusion in agar with the corresponding test system.
Indirect immunofluorescence
Weller and Coons (1954) tests were performed on tissue sections or monolayer of living cells from various murine organs.
Tissue blocks were frozen at –70° C and cut in cryostat at –20° C to give 3-µm sections. The sections were fixed in acetone at room temperature for 10 min. They were incubated with rabbit antibodies against Ag-Eb and donkey antibodies against rabbit IgG, conjugated with FITC (see Engelhardt, 1968).
A monolayer of living cells was obtained according to Dorfman (1970). Cell suspensions, prepared by careful disruption of tissues in Hanks' solution, were washed five times in the same solution. One drop of the final suspension was transferred from a capillary tube on a cover slide, pretreated with 1% formvar in dichlorethane. To obtain firm adherence of the cells to the formvar film the slides were incubated in a moist chamber at 37° C for 5-10 min. The adherent cells formed a monolayer and the rest of the suspension was discarded by washing in Hanks' solution. It is noteworthy that dead or damaged cells failed to adhere to the film so the monolayer obtained by this method, was formed practically exclusively by living cells.
All the antisera were predialysed against Hanks' solution. A drop of antibodies against Ag-Eb was placed on the monolayer, the incubation was carried out in a moist chamber for 20 min at 37° C, the slide was then washed carefully in five changes of Hanks' solution and incubated in the same way with FITC-conjugated donkey anti-rabbit IgG antibodies. After the same washing the cover slides were dried and placed on the microscope slide with 50% glycerol, buffered at pH 7.2-7.4. These preparations were examined in the fluorescence microscope ML-2. Two hundred cells were examined and the percentage of positive cells was calculated.
For morphological analysis sections, imprints and smears of cell suspensions were stained according to Romanovsky-Gimza. Sections were stained for 30 min, imprints and smears for 5 min.
Detection of antigen in plasma of mice with Rauscher virus-induced leukemia
The antigen, which has been termed the antigen of erythroblasts (Ag-Eb), was revealed by rabbit anti-sera to Rauscher virus. These antisera reacted in immunodiffusion with several antigens in plasma of mice with Rauscher leukemia: normal serum IgG and a2-globulin, MuLV-gs-1 (p30) and one non-identified antigen, Ag-Eb.
Some antisera to Rauscher virus possessed no antibodies to MuLV-gs-1 (p30). After neutralization with an equal amount of normal mouse serum such antisera continued to form one precipitation line with plasma of mice with Rauscher leukemia.
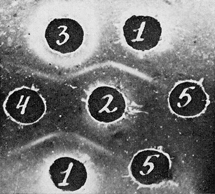
To study the corresponding antigen by immunodiffusion we prepared a specific test system, which consisted of the above-mentioned neutralized antisera to Rauscher virus and plasma of mice with Rauscher leukemia, concentrated by lyphogel to 1/2 of its original volume (Fig. 1).

| Figure 1
Comparison of partially purified Ag-Eb and antibodies to Ag-Eb with the corresponding test system. Test system for Ag-Eb. 1: Anti-sera against purified Rauscher virus, neutralized with equal amount of sera of normal mice; 2: plasma of BALB/c with Rauscher leukemia, concentrated to 1/2 of the original volume; 3: antibodies against Ag-Eb, isolated from specific precipitate; 4: electrophoretic fraction of spleen extract of mice with Rauscher leukemia; 5: saline. |
The antigen was regularly found in sera of BALB/c mice with Rauscher leukemia, while in sera or plasma of healthy BALB/c mice it could be revealed in trace amounts but only after concentration and not in all the samples.
Small but unmistakable amounts of the antigen were found with radioimmunodiffusion in preparations of Rauscher virus, similar to the immunizing material – fractions with density 1.14 and 1.16, obtained from plasma of mice with Rauscher leukemia after differential centrifugation in sucrose gradient (Fig. 2). However, the antigen could not be seen in commercial preparations of Rauscher virus.

| Figure 2
Ag-Eb in plasma preparation of Rauscher virus (immunoautoradiography). |
The antigen was found to be different from MuLV-gs-1 (p30) when the corresponding test systems were compared in immunodiffusion (Fig. 3). After immunoelectrophoresis of spleen extracts from mice with Rauscher virus-induced leukemia the monospecific antiserum to Ag-Eb revealed this antigen in the zone of serum β-globulins (Fig. 4), while the type-specific Rauscher leukemia antigen was found in a2-globulin zone (Abelev et al., 1968) and MuLV-gs-1 (p30) was characterized similarly by the mobility of γ-globulin (Engelhardt et al., 1969).

| Figure 3
Comparison of test system for Ag-Eb and MuLV-gs-1 (p30). Test system for Ag-Eb (the same as in Fig. 1): 1: antiserum; 2: antigen. |
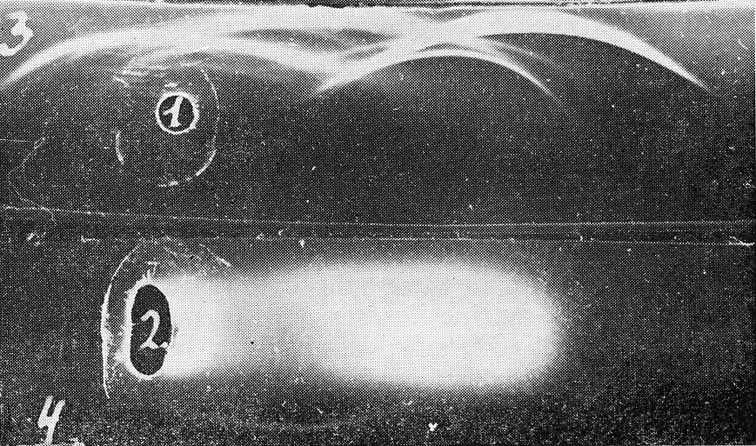
| Figure 4
Immunoelectrophoretic characteristics of Ag-Eb. 1: Normal mouse serum; 2: spleen extract from mice with Rauscher leukemia. Developed with rabbit antiserum against normal mouse serum (3) and with the absorbed antisera against Rauscher virus monospecific to Ag-Eb (4). |
The Ag-Eb-containing fraction, obtained after preparative electrophoresis in agarose, completely neutralized the precipitating (Fig. 1) and immuno-fluorescent activities (Fig. 6d) of the corresponding antibodies.
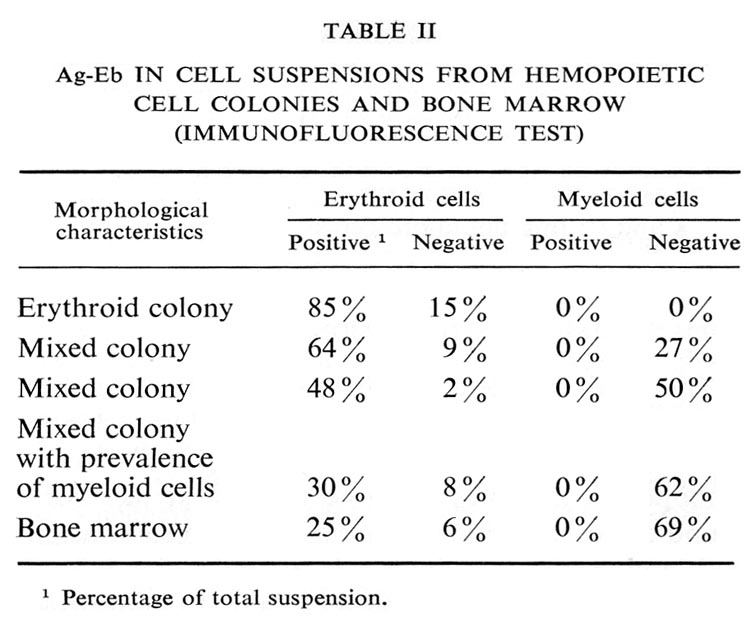
Presence of antigen in spleen extracts
The antigen was found in spleen extracts of all the tested mouse strains (Table I). It is noteworthy that with age its concentration decreased significantly.
To study the relationship of this antigen to fetal hemoglobin, four samples of fetal blood lysates were examined in immunodiffusion. Very slight deviation of the precipitin line of the test system for Ag-Eb was observed only in reaction with the most concentrated samples (Fig. 5).
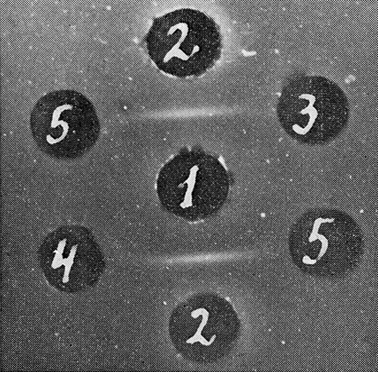
| Figure 5
Ag-Eb in the erythrocyte lysates from murine fetuses. Test-system for Ag-Eb: 1: antiserum; 2: antigen; 3: erythrocyte lysate (1.75 x l09 er/ml); 4: the same lysate as in 3, concentrated by 14 of the original amount; 5: saline. |
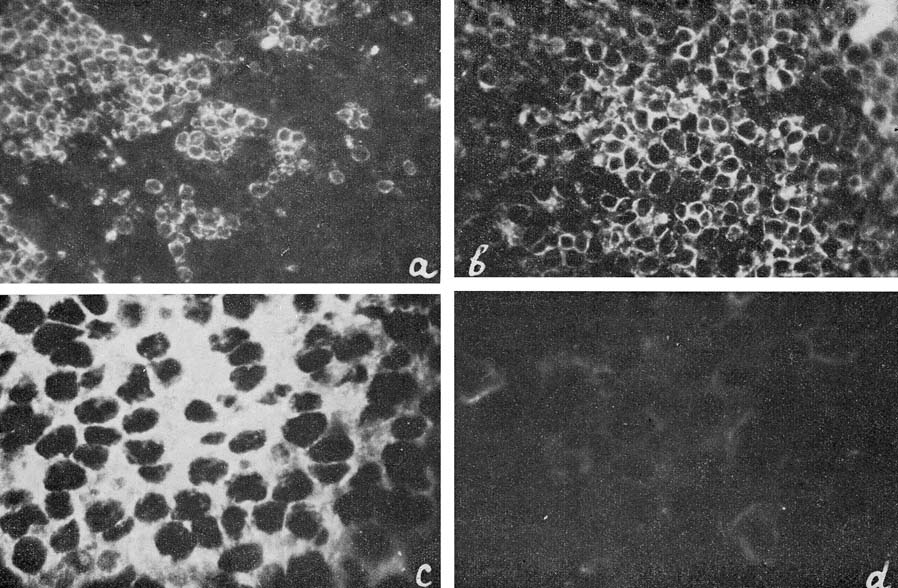
| Figure 6
a: Ag-Eb in spleen sections from healthy mice; b, c and d: from mice with Rauscher leukemia. |
Presence of antigen in various tumors
These data are summarized in Table I. The antigen was found only in spleen extracts from mice with leukemias, induced by Rauscher or Friend viruses. Both viruses are known to induce erythroblastosis.
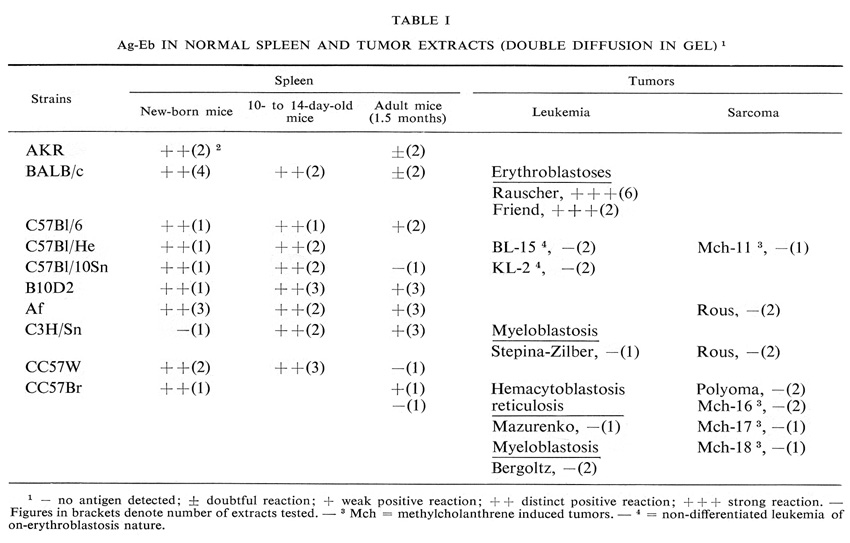
Immunofluorescent localization of the antigen
A problem of paramount importance was elucidation of the cellular origin of the antigen.
Bright membrane fluorescence was observed on the living cells of Rauscher leukemic spleens after incubation with absorbed antiserum monospecific for Ag-Eb in immunodiffusion, followed by treatment with FITC-conjugated anti-rabbit IgG antibodies. As the intact cell membranes are impermeable for antibody molecules, only surface antigens could be revealed by immunofluorescence on living cells. A similar, though appreciably weaker reaction, was observed with lymph-node cells. The reaction with the lymphoid cells disappeared after absorption with the corresponding cell suspension (2 x 109 cells per ml of the antiserum), while such treatment of the antiserum showed no influence on the fluorescence of the Rauscher leukemic spleen cells. In the following experiments ether-treated commercial Rauscher virus was added to this double-absorbed antiserum. Excessive amounts of RF and MuLV-gs-1 were revealed in it by immunodiffusion with the corresponding test systems. In spite of this the immunofluorescence reaction of the antiserum with Rauscher leukemic spleen cells remained unchanged.
The majority of the immunofluorescence studies were performed with antibodies to Ag-Eb, isolated from specific precipitate. These antibodies showed complete identity with the test system for Ag-Eb in immunodiffusion. Their precipitating and immunofluorescence activities coincided very well. A sample of the antibodies was neutralized with the electro-phoretic fraction, containing an equivalent quantity of Ag-Eb according to immunodiffusion (Fig. 1). No reaction was observed with neutralized antibodies on the tissue sections (Fig. 6d) or living cells, where distinct fluorescence was found upon incubation with active antibodies (Fig. 6a, b and c).
The antigen was found in the spleen sections of healthy BALB mice and in mice with Rauscher-virus-induced leukemia (Fig. 6a and b). In both cases the fluorescence was observed in the periphery of cytoplasm, the nuclei remaining dark. In normal spleen sections the antigen-containing cells were distributed in discrete groups (Fig. 6a), whereas in the sections of leukemic spleens the great majority of cells were positive (Fig. 6b and c).
We were unable to identify the cells which contained the antigen in normal spleen, on staining parallel sections after Romanovsky-Gimza. We therefore induced hemopoietic colonies in the spleens of irradiated mice according to the method of Till and McCulloch (1961). In one experiment mice were irradiated, in the second experiment the animals after irradiation were inoculated with a syngeneic bone-marrow cell suspension. In both cases 8-10 days later distinct discrete colonies were observed in the spleens. Some of these colonies were composed of erythroid cells, while the others contained both erythroid and myeloid cells.
The imprints of individual colonies were stained according to Romanovsky-Gimza for examination of their morphological characteristics.
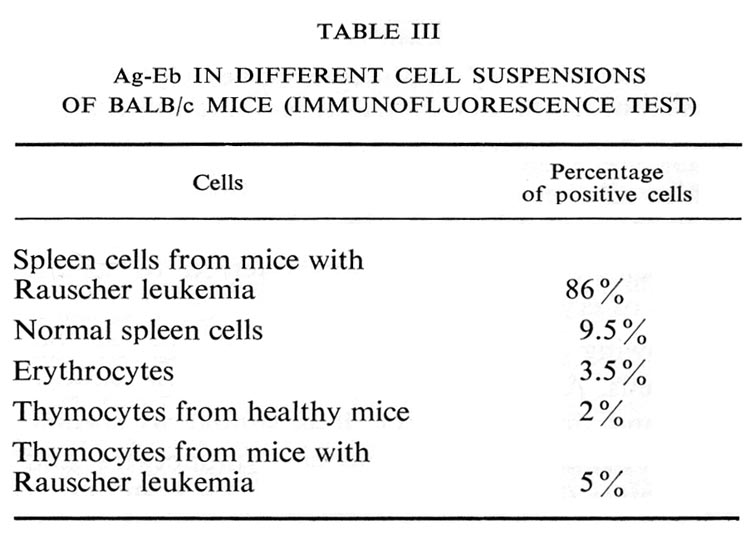
Living cell monolayers were prepared from individual colonies and studied by immunofluorescence. The results are summarized in Table II and illustrated in Figure 7.
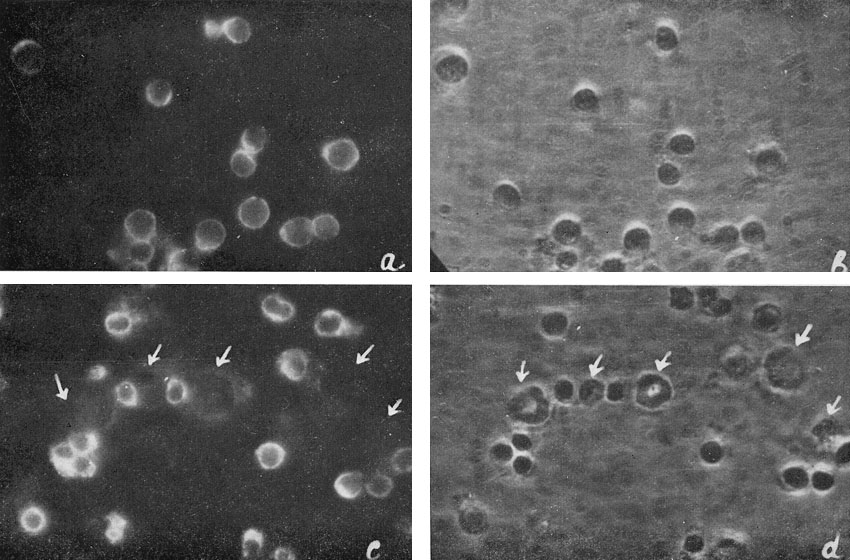
| Figure 7
Ag-Eb in cell suspensions from hemopoietic colonies: a and b : cell suspension from erythroid colony; c and d : cell suspension from mixed erythroid and myeloid colony. |
The antigen was never found on the myeloid cells. In the erythroid cell suspension 85% of cells were positive, in cell suspensions from mixed colonies there was a positive correlation between the percentage of erythroid and antigen-containing cells. All the cells containing the antigen were erythroblasts.
Similar results were obtained in the experiments with bone-marrow cell suspensions, i.e. all the positive cells were erythroblasts (Table II).
Several other cell suspensions tested displayed only occasional antigen-containing cells (Table III).
The antigen, detected in mice with Rauscher leukemia, seems to be peculiar to erythroblasts. Upon immunofluorescent study with living cells from hemopoietic colonies or bone marrow it has been found exclusively on erythroblasts. Its concentration in blood serum was higher in young animals and especially in mice with Rauscher and Friend virus-induced leukemia, which are both erythroblastoses. It is not identical with fetal hemoglobin, since only trace amounts of this antigen were found in concentrated lysates of fetal erythrocytes. It was not revealed in erythrocytes either in immunodiffusion or immunofluorescence.
Since all these data suggest that it is an antigen, specific for a certain differentiation stage of erythroid cells (i.e. erythroblasts) it was referred to as the antigen of erythroblasts – Ag-Eb.
Following immunofluorescent staining of the living cells, Ag-Eb is seen on the surface of erythroblasts and, in this respect, it is similar to specific surface antigens of thymocytes, lymphocytes and plasmocytes (Thy-1, TL, Ly-1, Ly-2, Ly-3, PC-1).
Ag-Eb differs from MuLV-gs-1 (p30) and type-specific Rauscher virus antigens by its immunological specificity and electrophoretic mobility. Moreover, it is not one of the obligatory viral components, for this antigen is absent in commercial preparations of Rauscher virus, grown in tissue culture.
Its presence in the preparation of Rauscher virus, isolated from plasma of leukemic mice, might be due to contamination of the virus with erythroblast debris, containing Ag-Eb. An alternative explanation is that the antigen is included in the virus envelope during its budding from erythroblasts. This problem could be solved using the immunoferritin technique with monospecific antibodies to Ag-Eb.
We are sincerely grateful to Dr. A. Ja. Fridenstein for suggesting the use of the hemopoietic cell colony technique and for his generous help in its use and to Dr. I. L. Chertkov (Institute of Hematology, Moscow) for the supply of irradiated mice and for his support in the second experiment. We wish to thank Dr. V. N. Stepina (IEKO AMS, Moscow), Dr. Z. A. Postnikova, Dr. I. N. Kryukova, Dr. B. D. Brondz, and Dr. I. S. Irlin (Gamaleya Institute, Moscow) for supplying leukemia and tumor tissues, Drs. A. I. Goussev and O. M. Lejneva for the MuLV-gs-1 test system, Mrs. A. E. Snegireva and Mrs. E. N. Rosinova for their help in histological studies and Dr. D. A. Elgort and Mrs. Yu. S. Hats for radio-immunodiffusion. The skillful technical assistance of
Mrs. M. D. Glishkina is gratefully acknowledged. The commercial preparation of Rauscher virus was kindly supplied by the Department of Resources and Logistics, NCI, Bethesda, USA, according to the USSR-USA agreement on collaborative studies in viral oncology.
The work was partly supported by the WHO Immunology Unit.
Chez les souris atteintes de la leucémie induite par le virus de Rauscher, un nouvel antigène a été mis en évidence. A cause de sa mobilité électrophoretlque et de sa spécificité immunochimique, cet antigène se distingue de l�antigene de Rauscher et de l�antigene spécifique de groupe des virus de la leucémie des souris. Cet antigène était présent dans le serum sanguin et dans l�extrait de la rate des souris normales de souches differentes. II a ete localise par la methode de Vimmunofluorescence sur la surface des erythroblastes mais pas sur les érythrocytes, lymphocytes, leucocytes et thymocytes. C'est pourquoi il a été nomme antigène des erythroblastes – Ag-Eb. L'Ag-Eb n'est pas identique á l�hémoglobine embryonnaire. II est évident que I'Ag-Eb est un marqueur spécifique d'un certain stade de I'érythropoièse.